Since the re-emergence of scleral lenses, optometrists prescribing them have grown at an exponential rate. According to the Contact Lens Spectrum Annual Market Share Review, scleral lens prescribing contributed approximately 5% of the overall contact lens market in 2022—up from 2% in 2021.1 Within the gas permeable (GP) lens market, scleral lens usage grew from 14% in 2021 to 21% in 2022.1 Even reports outside optometry predict growth of scleral lenses. Fortune Business Insights predicts the global scleral lens market to grow from $240 million in 2021 to $646.8 million by 2028.2 Many believe that several factors contribute to continued growth, including rising prevalence of ocular disorders, improving insurance coverage and reimbursement, and advancing technology that increase scleral lenses accessibility.2
Scleral lenses were originally viewed as a last resort therapy, reserved for the most severe cases of corneal ectasia or ocular surface disease. Patients who otherwise would require surgical intervention could wear scleral lenses, allowing them to forgo or delay corneal transplantation and other invasive, life-altering surgeries. But as the technology and usage have expanded, scleral lenses have become the first-line option for practitioners regardless of the condition or severity. Survey data reported has demonstrated this shift in prescribing trends among scleral lens practitioners, favoring scleral lenses over traditional corneal GP lenses for cases of corneal irregularity.3
While scleral lenses offer great opportunity—and undoubtedly their usage will only grow—they do come with unique complications and concerns. This article will review several potential complications one may encounter while working with scleral lenses, evaluating their clinical presentation and management.
 |
|
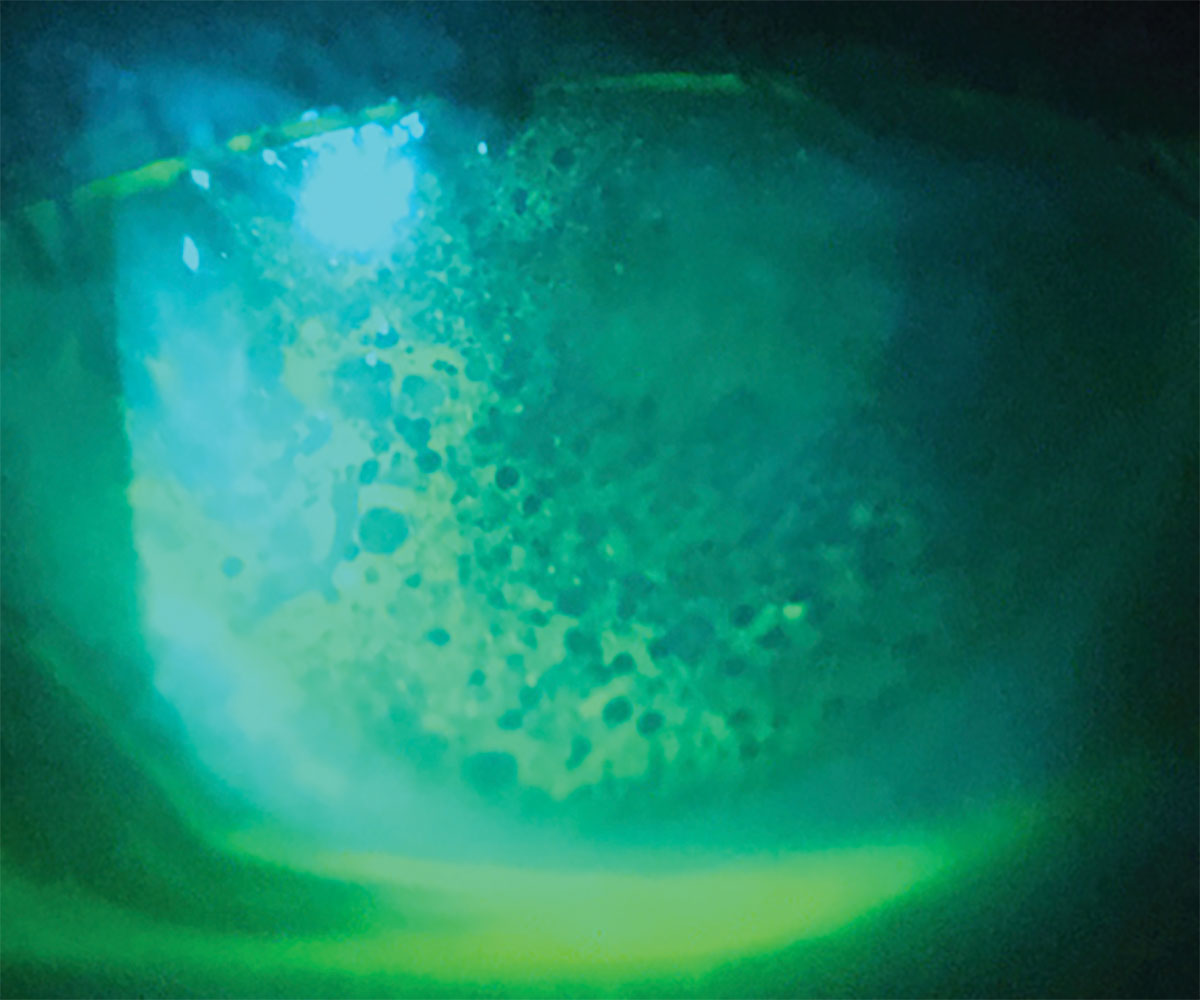
Fig. 1. Corneal edema presenting with microcystic edema and negative fluorescein staining. Click image to enlarge. |
Baseline Testing
For both the initial evaluation and long-term management of scleral lens wearers, certain baseline measurements are very beneficial to obtain. Corneal tomography or topography provides an assessment of the entire corneal shape that can aid in the initial scleral lens design, as well as tracks disease progression such as for patients with keratoconus. When available, scleral profilometry provides scleral shape analysis and thus can aid our scleral haptic design improving our success.
Corneal pachymetry should be obtained whenever possible, preferably global pachymetry rather than a single measurement point. By measuring and tracking the corneal thickness, one can track the functional capacity of the cornea specifically related to corneal edema. Specular microscopy is another helpful baseline to obtain, particularly when working with a patient who has a history of corneal transplant or endothelial dystrophy. By knowing an endothelial cell count, we can determine whether a patient may or may not be a good candidate for scleral lens wear. It is currently believed that endothelial cell counts below 800 cells/mm2 may be more susceptible to developing corneal edema and thus may not be good candidates for scleral lens wear.
Other useful imaging options include external photos for documentation of findings such as neovascularization, corneal opacities and conjunctival vessels, allowing for better future comparison. External photography of lenses can also be helpful when working with consultation on fit adjustments. Anterior segment OCT imaging is another helpful instrumentation that can provide high definition corneal and lens imaging to aid in fit assessment.
 |
|
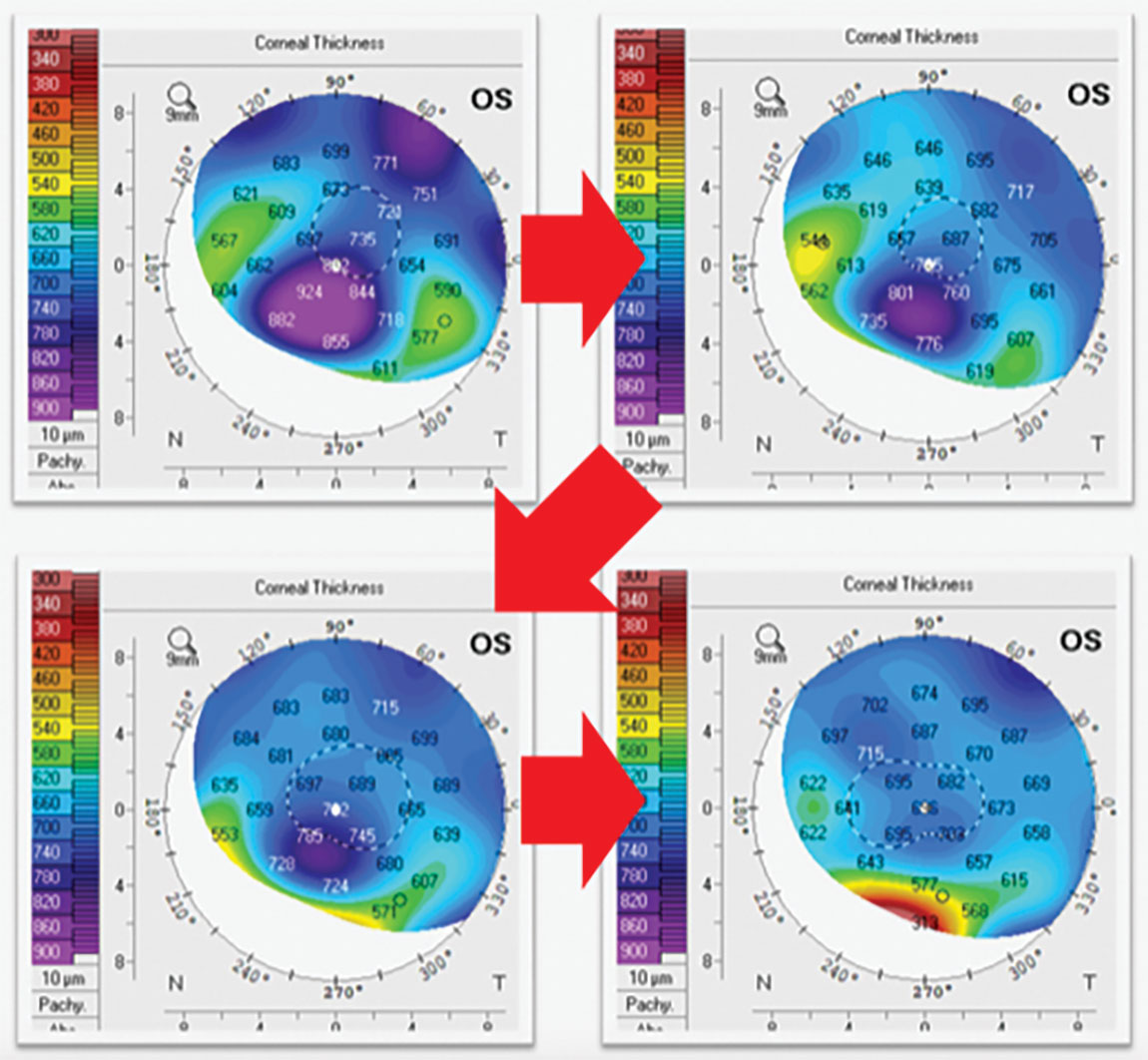
Fig. 2. Corneal pachymetry maps of a patient with corneal edema who was started on topical rho-kinase inhibitor showing gradual improvement on edema. Click image to enlarge. |
Corneal Edema
Possibly the most debated and controversial complication associated with scleral lens wear is corneal edema, accumulation of fluid within the cornea typically without inflammation. Currently, it is thought that a mild degree of corneal edema—less than 4%—occurs during daily scleral lens wear and resolves following lens removal.5 But in cases where the degree of edema surpasses this limit or fails to resolve following lens removal, we have a complication.
As corneal edema develops, patients may complain of blurred vision, halos around lights and photophobia. If the edema progresses to greater than 10%, patients may begin to experience pain and discomfort.4 Clinically, one may find an assortment of corneal changes including epithelial bullae or detachments, microcystic edema, corneal haze, stromal striae or Descemet’s folds, steepening of the corneal curvature and increased corneal thickness (Figure 1).
Corneal edema may develop as an underlying disease process such as keratoconus or Fuchs’ endothelial corneal dystrophy progresses, or it may develop as a result of scleral lens wear directly. The physical presence of a scleral lens may induce mechanical stress on the cornea through lens bearing or a tightly fitting lens leading to corneal edema. Similar to other contact lens modalities, scleral lens wear may result in corneal hypoxia that can lead to edema secondary to contact lens-induced endotheliopathy.5
While corneal edema may not always be avoidable, there are several steps that can be taken to limit its incidence. Initial evaluation with corneal pachymetry and specular microscopy can help determine if a patient is a scleral lens candidate. To avoid corneal hypoxia, choosing a high or hyper Dk material to maximize oxygen transmissibility is beneficial. Also minimizing lens thickness and post-lens tear reservoir thickness are advantageous goals that will help reduce the risk of hypoxia. During scleral lens fitting, avoiding limbal bearing and tight scleral landing zones will limit the mechanical stress of a lens on the eye. The use of fenestrations or channels within the lens design can improve corneal oxygenation through improved tear exchange as well as eliminate lens suction and adhesion.5
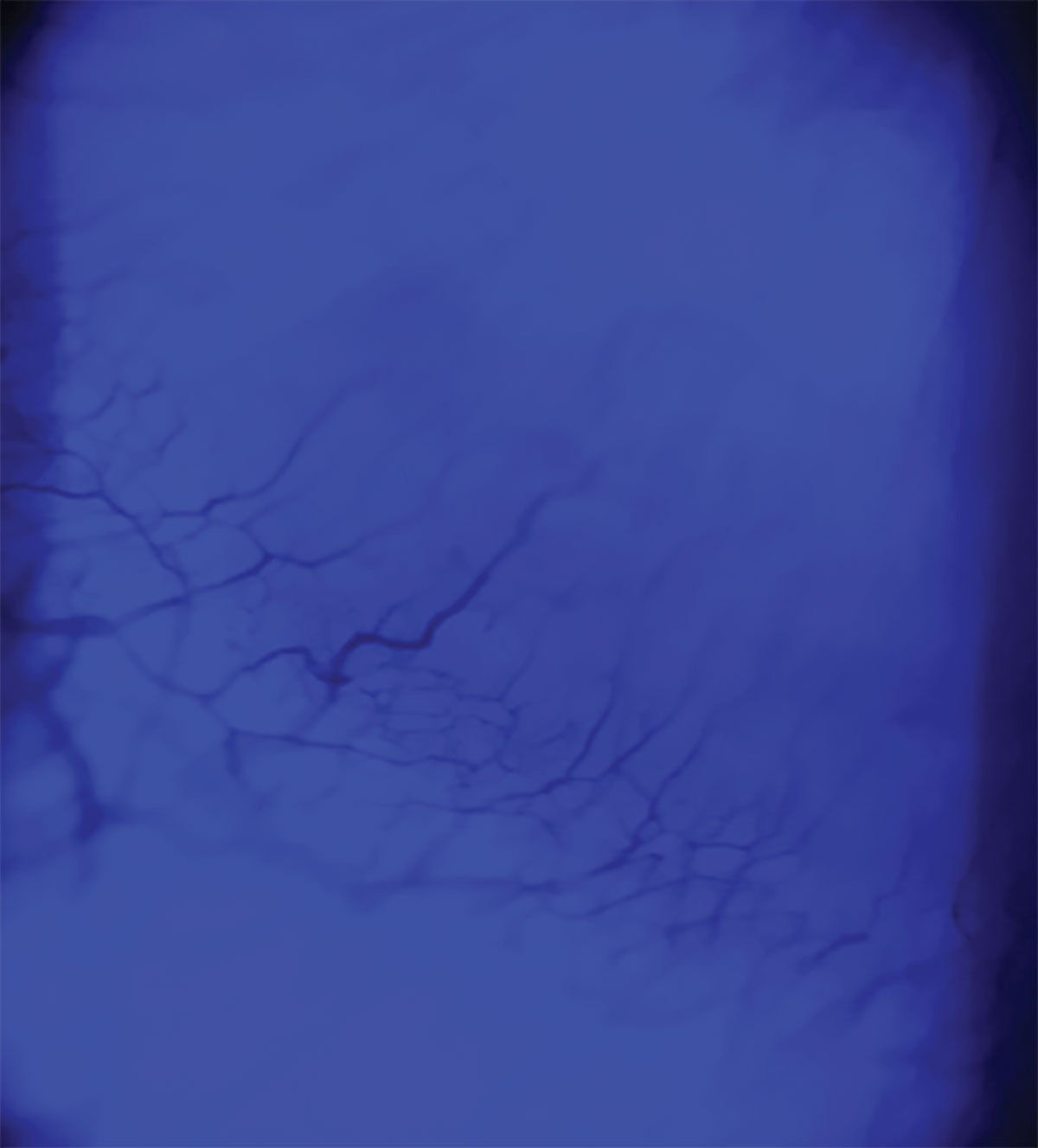 |
Fig. 3. Corneal neovascularization resulting from scleral lens extended wear visualized with cobalt light filter. Click image to enlarge. |
When corneal edema does develop, management should be dictated by patient symptoms and clinical findings. Mild and asymptomatic cases may benefit from reduced lens wear until proper lens adjustments are made to alleviate the causative stress, but for non-resolving or symptomatic cases of corneal edema, therapeutic intervention is warranted. Hypertonic solutions or ointments may be beneficial for more mild cases of edema but are difficult to use during scleral lens wear. Using prior to and following scleral lens wear may provide some reduction in corneal edema but typically has minimal effect. Patients with endothelial cell dysfunction rho-kinase inhibitors have shown to promote endothelial cell proliferation and suppress its apoptosis thereby improving endothelial cell count and function, providing a more long-term solution for corneal edema management.6 Take a look at the global pachymetry maps of this scleral patient, for example, post-corneal transplant (Figure 2). His transplant began to develop edema near the graft-host junction and eventually progressed to 924μm thick. He was then started on a topical rho-kinase inhibitor once daily and gradually the edema resolved.
Short-term management of symptomatic cases should include discontinuation of all lens wear, initiation of topical corticosteroid therapy, topical antibiotic if epithelial compromise is present and possible use of topical aqueous suppressant medications which can aid in reducing endothelial cell stress. For cases that are unable to recover with topical therapy, surgical intervention via endothelial keratoplasty may be warranted as well.
Another very concerning possible complication is corneal neovascularization, which results from the ingrowth of blood vessels from the limbal vascular plexus into the cornea (Figure 3).4,5 As it develops, the risk of corneal opacification increases, threatening the patient’s vision. Neovascularization can happen from scleral lens wear and is typically associated with corneal hypoxia, poor lens compliance or lens bearing.4,5
Depending on the severity of neovascularization, patients may be asymptomatic or complain of reduced vision as corneal clarity diminishes. Clinically, the presence of blood vessels extending into the cornea will be noted. More superficial blood vessels are commonly associated with hypoxia, while deeper, larger vessels are suggestive of inflammation, possibly related to an underlying disease process.
Maximizing corneal oxygenation by using highly oxygen permeable materials and optimizing lens and post-lens tear reservoir thicknesses again will aid in avoiding a hypoxic corneal state that may drive neovascularization. Pressure induced by limbal bearing or a tight scleral landing zone can trigger the development of neovascularization as well, thus lens adjustments should be made to minimize such mechanical stressors. Neovascularization may also arise from scleral lens overwear or extended wear, therefore emphasizing proper lens wear to patients is imperative to maintain proper corneal health.
 |
|
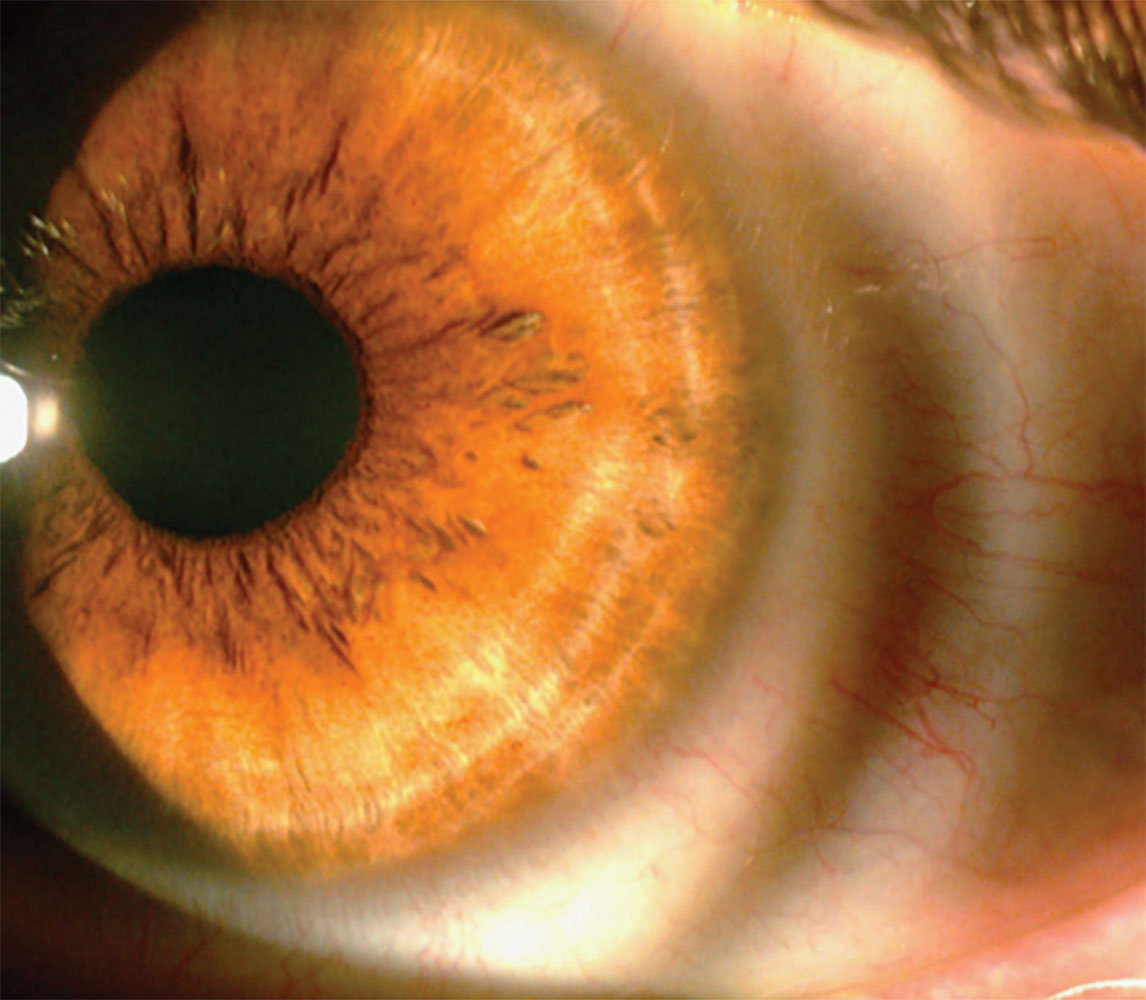
Fig. 4. Conjunctival compression ring following scleral lens removal in a tight fitting scleral lens. Click image to enlarge. |
Lens Bearing
When working with scleral lenses, sometimes a lens may settle more than expected over time or a patient’s condition may progress resulting in the lens directly resting on the cornea. The physical stress of a lens compressing the cornea can lead to corneal compromise and various complications depending on the region of bearing. Patient’s symptoms and clinical appearance may also differ depending on the area of bearing, dictating the proper lens modification.
Beginning peripherally, tight fitting lenses, which may be squeezing within the scleral landing or limbal zones, can result in complications such as corneal edema, neovascularization and keratitis. This is typically the result of a lens whose scleral landing zone is too steep, thus compressing the eye more than intended. Patients often will complain of discomfort, pain, photophobia, and pressure and/or tightness while wearing the lens. In many cases, these symptoms will worsen with prolonged wear and patients will report a sense of relief following lens removal.
Patients may struggle with lens removal due to the tightness and suction and complain of prominent conjunctival and/or limbal compression rings following removal (Figure 4). Lens evaluation will demonstrate blanching or impingement of the circumlimbal vasculature within the scleral landing zone and no lens movement. Adjusting the scleral landing zone to relieve the compression will solve the problem. If available, scleral topography or profilometry obtained prior to lens fitting can help avoid a tight-fitting lens.
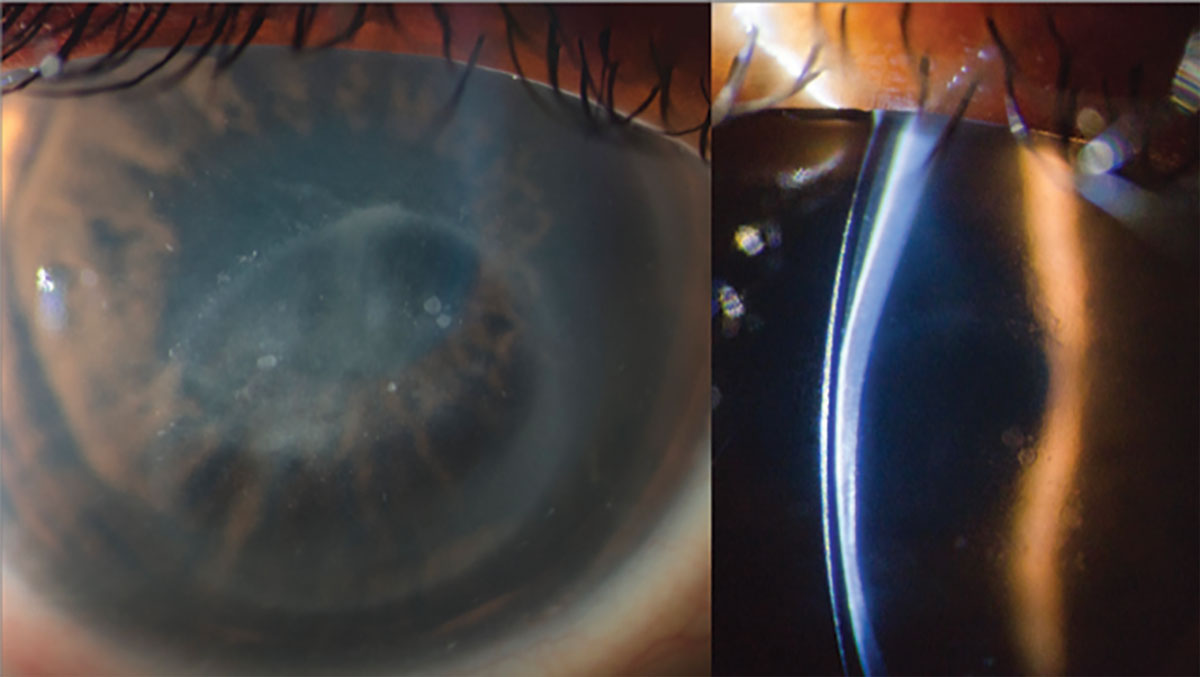 |
Fig. 5. Slit beam illumination demonstrating contact of a scleral lens with cornea (left). Direct visualization of scleral lens bearing on central cornea (right). Click image to enlarge. |
Moving inward, limbal bearing results when a scleral lens directly lands on the limbus and may again give rise to corneal edema, neovascularization, keratitis and limbal stem cell deficiency.5 Patients with more oblate shaped corneas such as post-refractive surgery and post-penetrating keratoplasty may be more susceptible to this due to the increased relative peripheral elevation. Because of this, assessing corneal diameter and corneal elevation prior to lens fitting can be helpful to ensure proper lens diameter and design are used to avoid this peripheral contact. When this does occur, patients may be asymptomatic or complain of discomfort, fatigue and pain similar to a tight-fitting lens. Following lens removal, a compression ring over the limbal/conjunctival area may be seen as well as circumlimbal punctate epithelial erosions and injection. To mitigate this issue, lens fit should be adjusted to reduce harsh bearing over the limbus by improving lens centration and increasing limbal clearance.
Central bearing occurs more commonly with prolate shaped corneas such as keratoconus and other ectatic conditions. Again, unexpected lens settling or progression of a patient’s underlying condition can lead to this unwanted contact. Patients may complain of reduced acuity, foreign body sensation and pain that is worse after scleral lens removal. Lens evaluation will reveal an area of lens-cornea contact that can be visualized with both direct illumination and slit beam (Figure 5). The cornea underneath is best evaluated with sodium fluorescein and may demonstrate an epithelial swirl pattern that results from lens compression in that area. Harsher examples of bearing can erode through the epithelium resulting in a corneal abrasion, which may be associated with infiltrates and stromal haze. In such cases, discontinuation of lens wear and therapeutic management with topical antibiotic and corticosteroid drops until resolution of the abrasion should be done. Once corneal compromise has been managed, the lens fit should be adjusted to increase sagittal depth and provide proper clearance. For cases of progressed corneal ectasia, referring the patient for corneal cross-linking to help avoid any further progression is warranted.
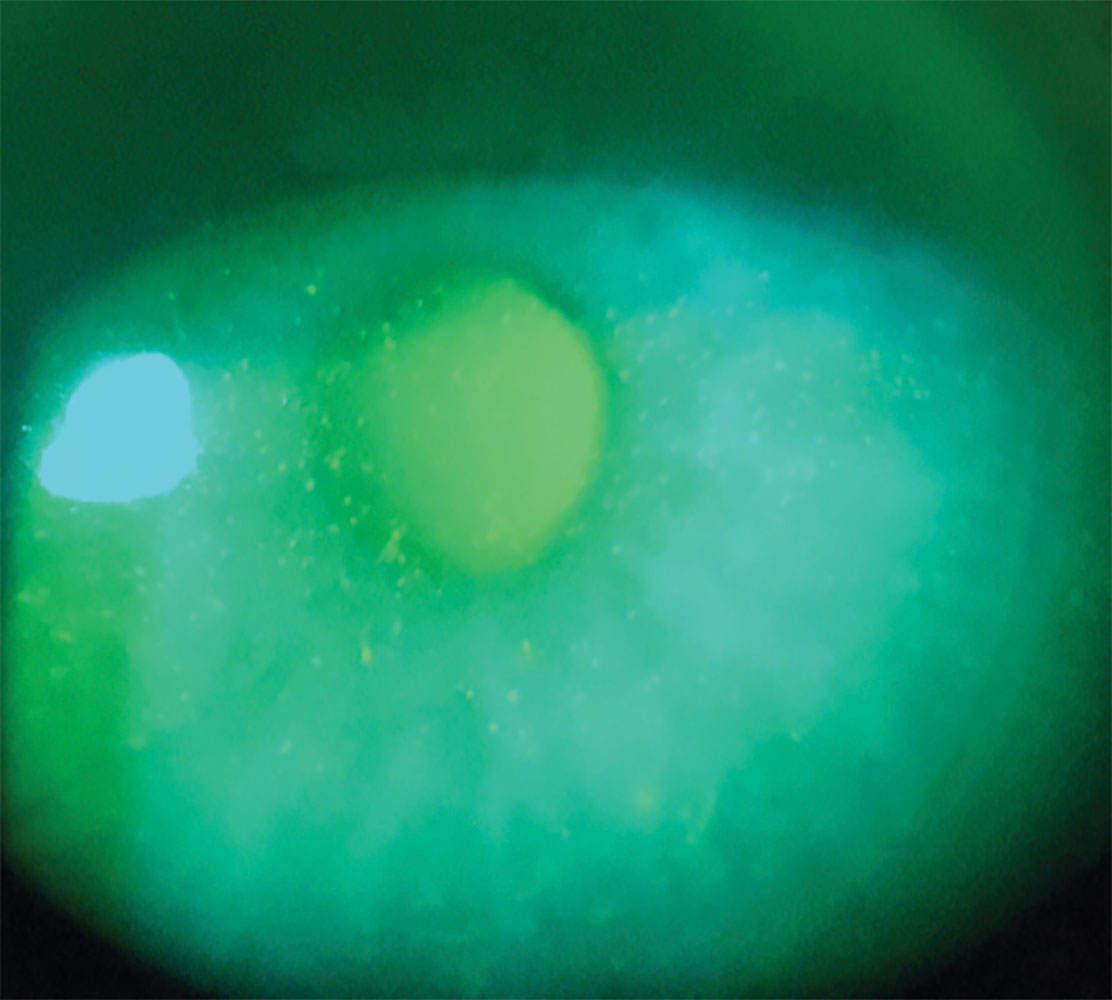 |
|
Fig. 6. Punctate epithelial erosions from a patient wearing a scleral lens filled with multipurpose soft contact lens solution. Click image to enlarge. |
Toxic Epitheliopathy
With the post-lens tear reservoir bathing the corneal epithelium completely during scleral lens wear, proper lens filling solution is required. Preservative-free saline or preservative-free artificial tears are commonly agreed upon as the ideal options for scleral lens filling; however, patients may forget or encounter instances where they are without one of those options giving rise to toxic epitheliopathy. This can range from mild sensitivity reactions to more severe chemical burns depending upon the offending agent. Mild reactions often are asymptomatic while more severe cases can present with pain, photophobia and blurred vision. Mild cases occur most commonly and often include instances of patients using various multiple purpose contact lens solutions or preserved artificial tears to fill their lenses for application. This can also occur in patients who use more viscous GP lens solutions to store their scleral lenses in and are not thoroughly rinsing the scleral chamber prior to application. Clinically, patients may present with diffuse punctate epithelial erosions, ranging from one to four more typically with a uniform distribution (Figure 6). Prescribing appropriate lens filling solutions and preparation techniques when first dispensing lenses and reviewing at follow-up appointments can help remind patients what solutions they should use. More severe cases need to be treated as a chemical burn and possible ocular emergency. Identification of the offending agent and its pH will drive the course of management.
 |
Fig. 7. Limbal epithelial bogging and epithelial wrinkling. Click image to enlarge. |
Epithelial Bogging
Visible following lens removal, epithelial bogging presents as a water-logged appearance of the peripheral epithelium (Figure 7).4,5 Best visualized following fluorescein application, one may find raised, irregular epithelium and circumlimbal punctate stippling. Patients are typically asymptomatic, and this condition is considered benign, resolving with continued wear and adaptation of the epithelium to the new environment. If after continued wear it has not resolved, adjusting the prescribed scleral lens filling solution may be necessary. Patients could also use a name brand solution compared to generic, or a buffered vs. non-buffered formulation. In some instances, using a formulation that contains electrolytes to mimic the natural tears more closely may be beneficial for patients.7
Conjunctival Prolapse
This occurs when redundant, loose conjunctiva is drawn into the transition or limbal zone under the lens (Figure 8), believed to be the result of negative pressure forces.4,5 Conjunctival prolapse is more commonly seen in older patients who naturally have more loose conjunctival tissue, but can happen within any age group. It’s typically considered a benign finding, but in some circumstances can become problematic. Should enough tissue be drawn into the scleral chamber, it could interfere with vision and thus require intervention. In cases of persistent conjunctival prolapse, corneal neovascularization has been noted underneath the conjunctival flap, so careful monitoring with lens removal and thorough corneal evaluation is indicated. For problematic conjunctival prolapse, decreasing limbal clearance can help reduce the negative pressure force drawing the tissue into that area, thereby reducing or eliminating it. In some cases, anti-inflammatory therapies including cyclosporine, liftegrast, topical corticosteroid pulse or amniotic membrane therapy can help to tighten the conjunctival tissue as well. When there is excessive amounts of conjunctivochalasis, surgical resection could be considered.
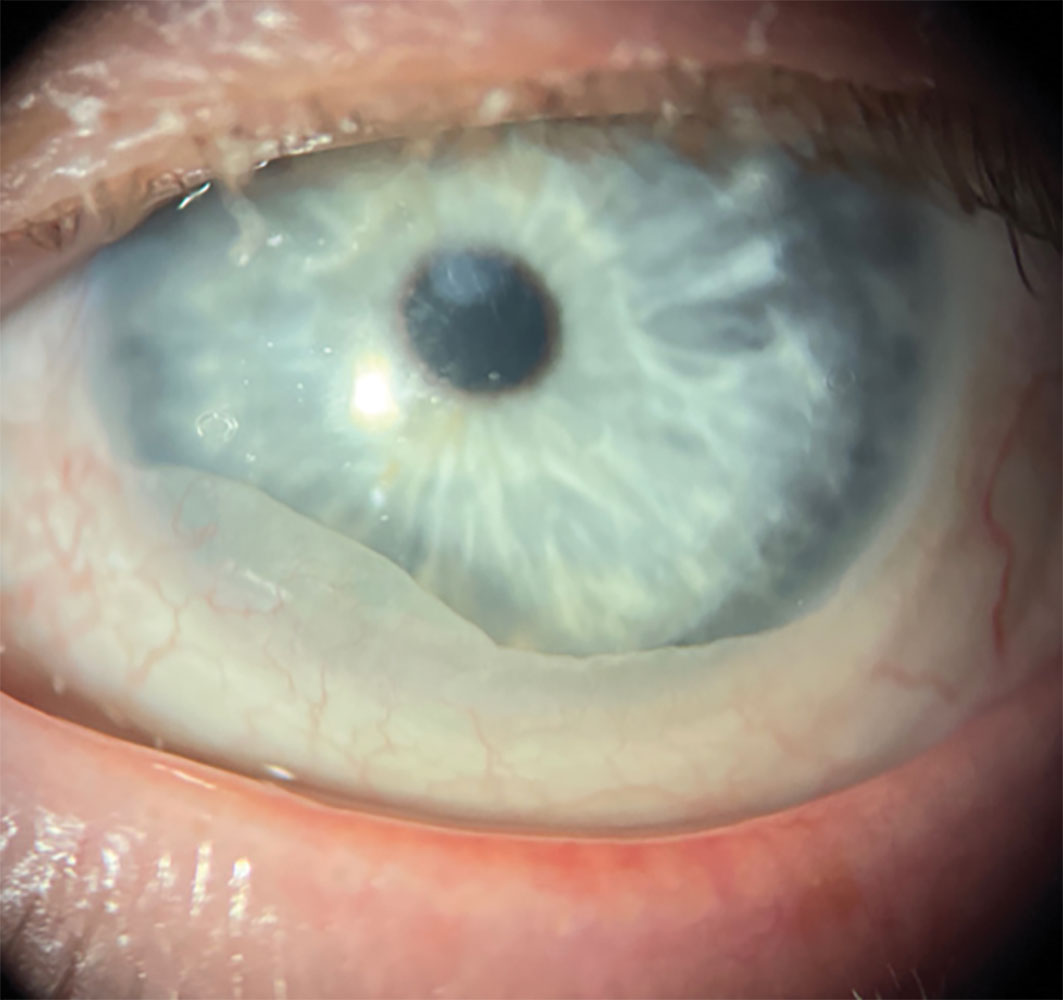 |
Fig. 8. Limbal epithelial bogging and epithelial wrinkling. Click image to enlarge. |
Takeaways
While scleral lenses come with their own unique array of potential complications, mitigating them typically comes down to three goals: maximizing corneal oxygenation, ensuring proper lens fit and emphasizing proper lens compliance. Implementing these fundamental traits when fitting scleral lenses can help one avoid potential complications or drive appropriate management should they arise.
Dr. Fosso serves as the director of contact lens services at PineCone Vision Center in Sartell, MN. He joined the practice in 2017 after completing a residency in anterior segment disease and medical contact lens management at Davis Duehr Dean in Madison, WI. He lectures and writes on topics of anterior segment disease management, surgical comanagement and contact lenses, with a special interest in keratoconus and scleral lenses.
1. Nichols JJ, Fisher D. Contact lenses 2022. CL Spectrum. 2023;38:20-2,24-6. 2. Scleral lens market size, share & COVID-19 impact analysis by type, by end user and regional forecast, 2021-2028. Fortune Business Insights. https://www.fortunebusinessinsights.com/scleral-lens-market-106557. March 1, 2022. Accessed August 24, 2024. 3. Shorter E, Fogt J, Nau C, et al. Prescription habits of scleral lenses for the management of corneal irregularity and ocular surface disease among scleral lens practitioners. Eye Contact Lens. 2023;49(2):46-50. 4. Fadel D. Scleral lens issues and complications: their recognition, etiology and management. Dougmar; 2020. 5. Barnett M, Johns LK. (2017). Ophthalmology: current and future developments: contemporary scleral lenses theory and application (4th Vol). Bentham Science Publishers; 2020 6. Singh NK, Sahu SK. Rho-kinase inhibitors: Role in corneal endothelial disorders. Seminars in Ophthalmology. 2022;38(1),9-14. 7. Satjawatcharaphong P. The scleral lens vault: saline overview. CL Spectrum. 2018;36:46. https://www.clspectrum.com/issues/2021/march-2021/the-scleral-lens-vault. |


