In certain cases, fitting scleral lenses is challenging, as they can induce edema and compromise corneal health. Corneal swelling when wearing these lenses may be caused by multiple factors, such as hypoxic stress, increased intraocular pressure (IOP), compromised functional condition of the endothelium and lens suction. Therefore, lens material selection, lens fitting and customization and ocular health monitoring are crucial to prevent or manage edema.
Lens Material Selection
Included in this category are considerations of oxygen permeability (Dk), lens thickness, lens hardness and resistance to flexure and deposits formation. Theoretical models have shown that a scleral lens made of materials with a Dk higher than 100, a thickness less than 260μm and a vault less than 150μm prevents corneal swelling related to hypoxia.1 Another theoretical approach showed opposing results: a lens with Dk 100 and a central clearance of 1000μm could provide more than sufficient oxygen to the cornea, considering 20% of tear mixing under the lens.2 Several clinical studies also show that corneal edema was nonsignificant with a lens vault higher than 400μm.1 Recently, however, a study compared contact lenses made with different Dk materials, ranging from 65 to 180, with the same lens thickness. Results indicated no statistically significant difference in corneal edema between each of the scleral lenses with Dk from 100 to 180.3
 |
|
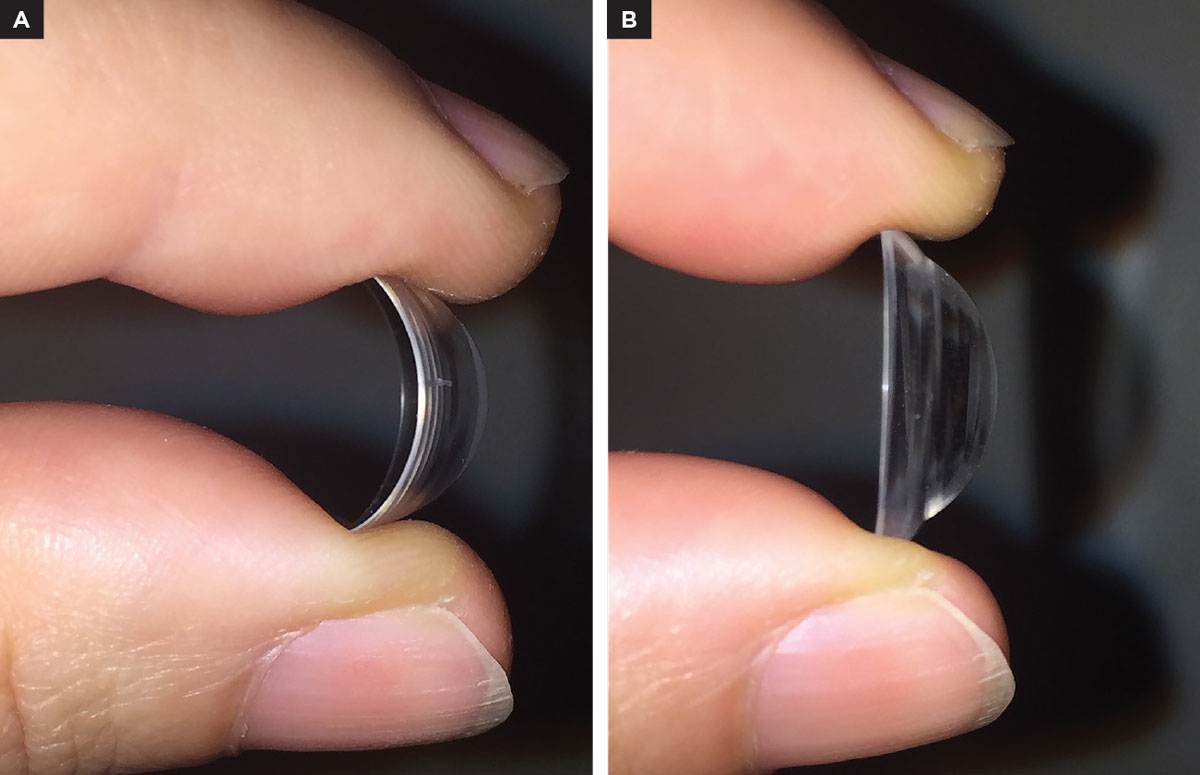
Fig. 1. (A) High Dk scleral lens flexing. (B) A scleral lens of the same diameter and thickness but with higher modulus. Click image to enlarge. |
Special considerations should be addressed for compromised corneas (e.g., post-keratoplasty, endothelial abnormalities, epithelial defects). In these cases, a Dk of more than 100 is indicated. Generally, by increasing the Dk the lens flexure will also increase, as the material has a lower modulus rating than low Dk materials (Figure 1). Therefore, choosing a material with resistance to flexure with a higher modulus rate is important. Also, the hardness of the material needs to be higher. The harder it is, the higher the material’s stiffness. Flexure also depends on lens design; a better alignment with the underlying conjunctiva may reduce lens flexure in the eye. If lens flexure persists, lens thickness may need to be increased.
Lens Design and Vault
These deal with Dk and the thickness of the post-lens tear layer. Tear permeability has a Dk value of approximately 80, which is relatively low compared to some high Dk materials.4,5 Additionally, when fitting scleral lenses, the tear exchange at lens settling is limited to 0.2% per minute.6 However, the tear exchange rate may vary with lens fitting and be reduced or increased by altering the lens periphery. Additionally, no studies have shown clinically significant levels of hypoxia during daily wear of sclerals, even in cases with oxygen permeability below Holden-Mertz criteria (87). Even if the ideal vault is yet to be determined, it is appropriate to fit a scleral lens with minimal clearance height in compromised corneas to prevent corneal swelling.
 |
|
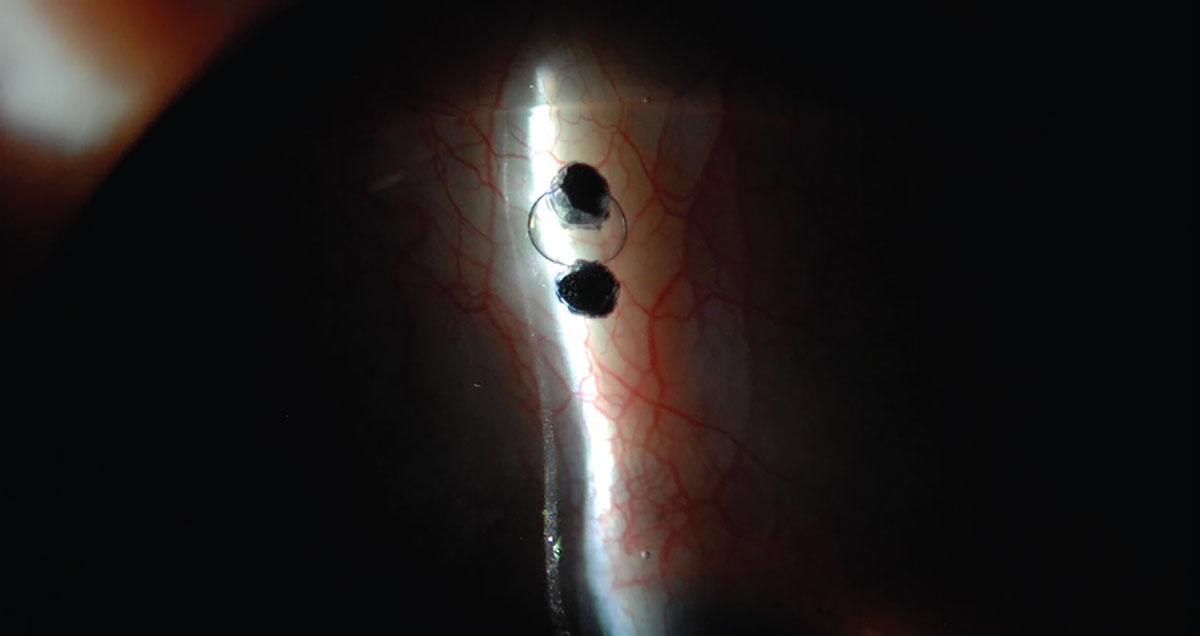
Fig. 2. Two fenestrations are included in the limbal area at two o’clock and ten o’clock. Click image to enlarge. |
Other Fitting Factors
When wearing scleral contact lenses, edema may occur due to aspects unrelated to hypoxia. Epithelial edema has been reported as an effect of lens suction on the eye.7,8 Suction occurs due to prolonged contact between the lens and the ocular surface and can increase with conjunctival swelling or a significant pressure of the lids exerted during blinking.7 If all of these occur, there appears to be a force to increase fluid accumulation within the epithelial epithelium.9 To reduce lens suction, fenestration in the limbal area or the lens periphery, peripheral channels in the scleral zone or loosening the fit in one or more quadrants may be indicated.
A tight fit and limbal congestion may increase the risk of lens suctioning. Increasing lens diameter and/or flattening the fit by reducing the corneal and limbal clearance resolves this issue. Fenestrations, channels and loosening the fit in the lens periphery may also reduce lens tightening on the eye and limbal congestion.
Fenestrations
One or more fenestrations may be included in the lens and can be positioned in the limbal area or the lens’s scleral zone.
Limbal area. The hole in the limbal area has several benefits, including reduced lens suction.10 To avoid air bubble formation, the hole must have a diameter of 0.3mm to 0.5mm and a limbal clearance of 75μm.10 Additionally, the lens’s back surface should relate as closely as possible to the contour with central clearance not exceeding 150μm. If the diameter of the hole is kept small (not greater than 0.5mm) when filling the lens with preservative-free saline, the solution will not go through the holes and empty the bowl. This is demonstrated in Video 1 (see above).
|
|
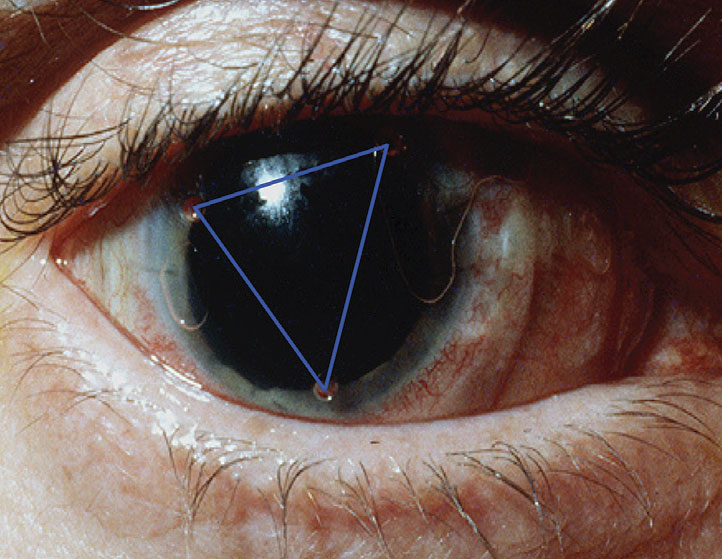
Fig. 3. Three fenestrations are included, at one o’clock, six o’clock and ten o’clock. The three holes create a triangle. Photo: Don Ezekiel, Dip. Opt (WA). Click image to enlarge. |
By appropriately controlling the hole size, the post-lens fluid reservoir releases through the hole when the lens is applied to the eye. This is due to negative sub-atmospheric pressure created behind the lens. Fenestrations are either ordered from the lab or self-made, demonstrated in Video 2 (see below).
Once the best fit is achieved and the lens is stable on the eye and not rotating, the fenestrations may be included. They need to be placed in an area that is uncovered by the eyelids. If two fenestrations are included, the best position for them could be at the two o’clock and ten o’clock positions (Figure 2). If a third hole is needed, though, its position may be at five o’clock or seven o’clock, so that the three holes create a triangle. The third hole may also be included at six o’clock if it is not covered by the lower eyelid (Figure 3). If the hole is self-made, it is necessary to mark the position when the lens is applied to the eye to ensure its correct placement.
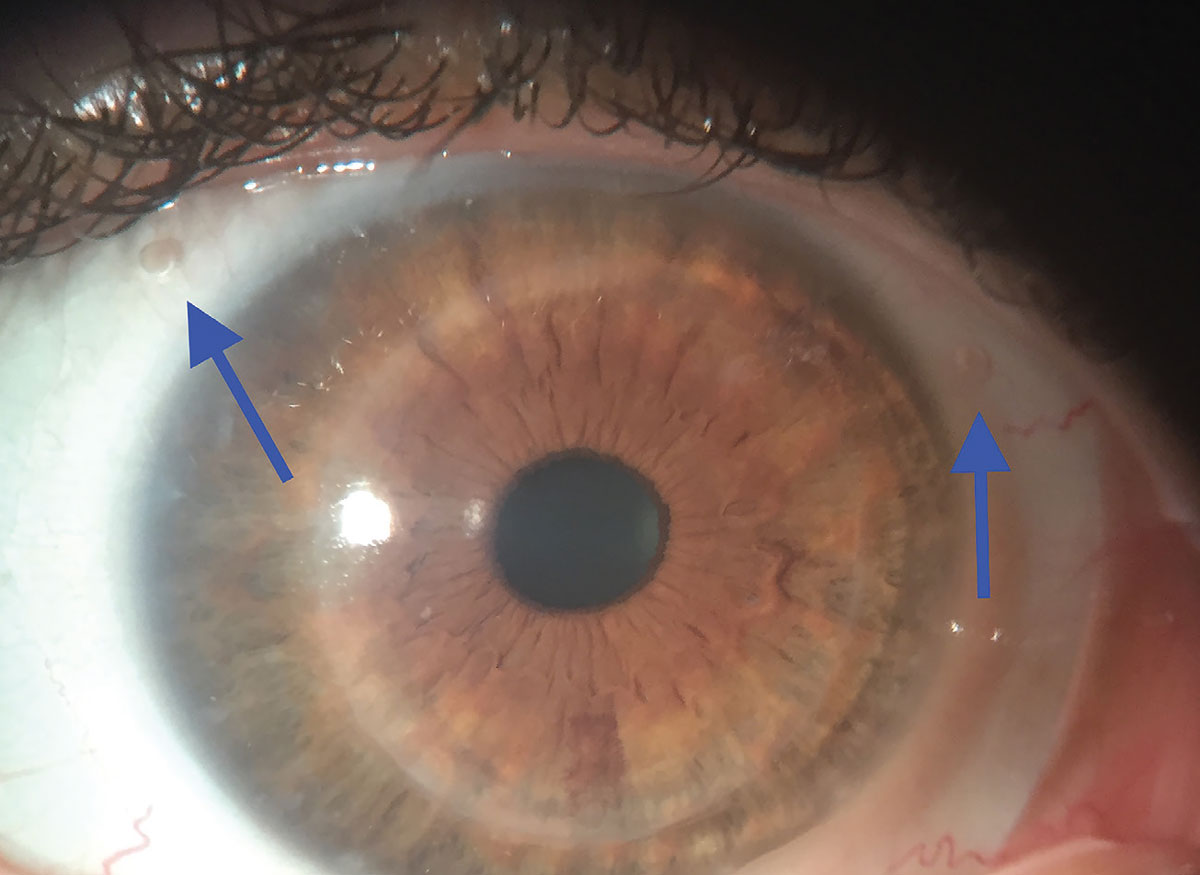
Scleral area. The fenestration may also be included in the scleral zone to relieve lens suctioning (Figure 4). In this case, the lens needs to be flattened where the hole is included to avoid conjunctival tissue penetrating the hole and occluding it, which would result in impeded functioning.
 |
|
Fig. 4. A fenestration included in the scleral area of the lens. Photo: Karen Carrasquillo, OD. Click image to enlarge. |
Channels
Peripheral channels to prevent or minimize lens suction was described as early as 1968.11 Channels will also allow a tear exchange, promoting corneal health (Figure 5). If lens suctioning persists, all this technology may be included in the same lens, including fenestrations in the limbal area, as well as in the scleral area and channels (Figure 6).
 |
|
Fig. 5. Channels are included to reduce and avoid lens suction. Photo: Karen Carrasquillo, OD. Click image to enlarge. |
IOP
It has been reported that corneal edema is correlated to an increase in intraocular pressure (IOP). Epithelial edema appears as soon as IOP significantly increases and progresses as IOP rises.12,13
Recent studies have found similar results, showing that an IOP increase causes damage to the endothelial cells and their density, provoking edema.14,15 Even though IOP was not demonstrated to be exceedingly high with scleral lens wear, it is essential to monitor IOP at follow-up visits and eventually manage its increase.16
Hypertonic Solution Use
Severinsky et al. proposed using hypertonic saline (5% NaCl) to accelerate the recovery of edema when it occurs. If edema persists, a topical 5% NaCl solution may be used during scleral lens wear.17 However, exercise caution when using hypertonic solutions as they may foster the survival of Staphylococcus aureus.18
Wear Time
If corneal edema persists despite changing lens material, optimizing lens fit and monitoring IOP and osmolarity, reduced wear time may be indicated in patients who desperately need their lenses, although only for a few hours per day. Scleral lenses may be worn for four hours daily, allowing edema to recover during the following awakening hours.
 |
|
Fig. 6. Fenestrations in the limbal and scleral areas as well as channels were all included to reduce lens suction. Photo: Karen Carrasquillo, OD. Click image to enlarge. |
Takeaways
While corneal edema may occur when wearing scleral contact lenses, there are different ways to mitigate this risk. Strategies like selectively picking lens material, lens thickness, lens design and fitting, customization including fenestrations and channels and monitoring IOP can especially help in patients at higher risk of its increase. If edema persists, hypertonic saline may be used or wearing time may be reduced, allowing corneal thickness recovery.
Dr. Fadel is a clinical scientist at the Center for Ocular Research and Education (CORE). She is a pioneer of modern lens designs and a specialist of contact lenses for irregular corneas, scleral lenses, myopia control and orthokeratology. She is Editor-in-Chief of the Journal of Contact Lens Research & Science. Dr. Fadel is a fellow of the Scleral Lens Education Society, the British Contact Lens Association, the American Academy of Optometry and the International Association of Contact Lens Educators.
1. Fadel D. Scleral lens issues and complications related to a non-optimal fitting relationship between the lens and ocular surface. Eye Contact Lens. 2019;45(3):152-63. 2. Bergmanson JP, Ezekiel DF, van der Worp E. Scleral contact lenses and hypoxia: Theory versus practice. Cont Lens Anterior Eye. 2015;38(3):145-7. 3. Dhallu SK, Huarte ST, Bilkhu PS, et al. Effect of scleral lens oxygen permeability on corneal physiology. Optom Vis Sci. 2020;97(9):669-75. 4. Benjamin WJ, The Dk Reference Study Group. Revised oxygen permeability (Dk) of reference materials. Invest Ophthalmol Vis Sci. 2006;47(13):ARVO E-Abstract97/B385. 5. Fadel D. Modern scleral lenses: mini versus large. Cont Lens Anterior Eye. 2017;40(4):200-7. 6. Vance KD, Miller W, Bergmanson J. Measurement of tear flow in scleral contact lens wearers. Poster presented at the American Academy of Optometry meeting, 2015. 7. Miller D, Carroll JM. Corneal edema and scleral lenses. Int Ophthalmol Clin. 1968;8(3):623-35. 8. Ridley F. Trans Ophthal Soc U.K. 1964;68:385. 9. Carrasquillo K, Byrnes S. Corneal edema and sclera lenses. Cont Lens Spectrum. 2018;33:34-41. 10. Fadel D, Ezekiel DF. Fenestrated scleral lenses: Back to the origins? Review of their benefits and fitting techniques. Optom Vis Sci. 2020;97(9):807-20. 11. Rosenthal P. Corneal contact lenses and corneal edema. Int Ophthalmol Clin. 1968;8(3):611-21. 12. Ytteborg J, Dohlman CH. Corneal edema and intraocular pressure. I. Animal experiments. Arch Ophthalmol. 1965;74:375-81. 13. Ytteborg J, Dohlman CH. Corneal edema and intraocular pressure. II. Clinical Results. Arch Ophthalmol. 1965;74:375-81. 14. Melamed S, Ben-Sira I, Ben-Shaul Y. Corneal endothelial changes under induced intraocular pressure elevation: a scanning and transmission electron microscopic study in rabbits. Br J Ophthalmol. 1980;64(3):164-9. 15. Urban B, Bakunowicz-Łazarczyk A, Michalczuk M, Krętowska M. Evaluation of corneal endothelium in adolescents with juvenile glaucoma. J Ophthalmol. 2015;2015:895428. 16. Fadel D. Kramer E. Potential contraindications to scleral lens wear. Cont Lens Anterior Eye. 2019;42(1):92-103. 17. Severinsky B, Behrman S, Frucht-Pery J, Solomon A. Scleral contact lenses for visual rehabilitation after penetrating keratoplasty: long-term outcomes. Cont Lens Anterior Eye. 2014;37(3):196-202. 18. Cho P, Boost M. Rivers and mountains may change, human nature does not! (traditional Chinese saying). Cont Lens Anterior Eye. 2009;32(4):155-6. |