Dry eye, like many other conditions, has been extensively studied for decades. As the cases of symptomatic patients continue to increase, a considerable amount of research has been done to better understand the ocular surface and tear film.
In 1995, the National Eye Institute provided one of the first global definitions, etiologies and classifications of dry eye. At that time, dry eye was defined and recognized as a “disorder of the tear film due to tear deficiency or excessive tear evaporation.”1 Since then, the definition has been reviewed and revamped through better understanding of the pathophysiology. A recent tear film subcommittee DEWS II report added changes to the definition to state that dry eye is a “multifactorial disease” that results in tear film instability, inflammation, hyperosmolarity of tears and the effect neurosensory components have on dry eyes.2
The tear film covers the cornea, bulbar and palpebral conjunctiva and is composed of three layers—the lipid, aqueous and mucin layers, although the TFOS DEWS II report has argued for combining the aqueous and mucin layer into the muco-aqueous layer.3 The disruption to any of these layers or a combination of them will lead to an abnormal and unhealthy ocular surface.
There are several functions of the tear film, including maintaining a smooth surface for refraction of light, lubrication of the ocular surface, supplying the avascular cornea with nutrients, removing foreign materials from the cornea and conjunctiva, and protecting the eye from pathogens.4
Tear film instability can be associated with a wide range of factors. Abnormalities or problems with the tear layer lead to loss or insufficient supply of tear film to environmental and external factors such as incomplete lid closure, malposition or lagophthalmos. An incomplete blink or lid malposition leads to inadequate lipid distribution over the tear film and areas of exposure have increased evaporation. Therefore, external factors including long-term contact lens wear, increased hours or prolonged use of digital devices can also lead to lipid deficiency.5
Here, we will discuss the pathophysiology of factors that affect the tear film. Proper knowledge of what causes patients’ symptoms will help make you make the right decisions on treatment for each specific patient.
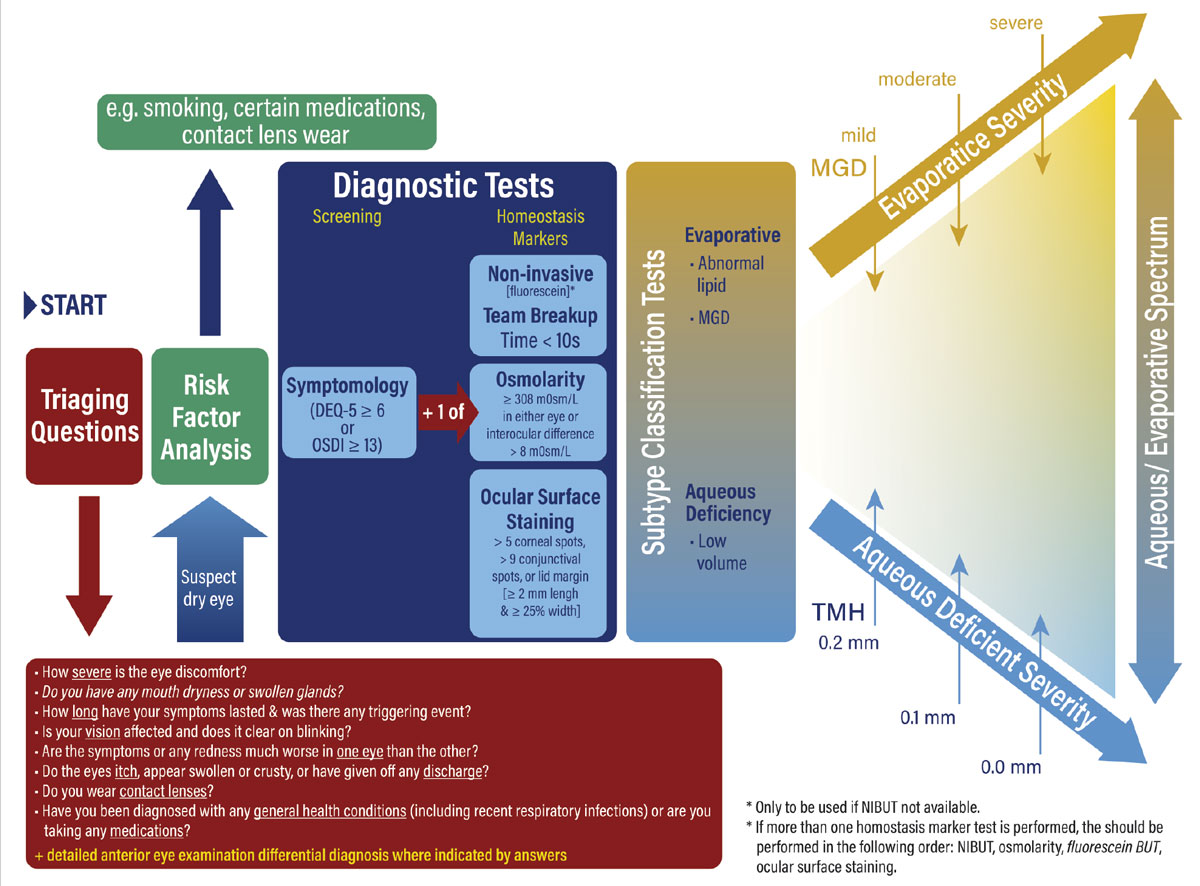 |
| The TFOS DEWS II dry eye diagnostic algorithm at a glance. Click image to enlarge. |
Lipid Layer
This is the outermost layer that serves as the connection and barrier from the ocular surface to the environment. Besides serving as a barrier, the lipid layer provides tear film stability by reducing surface tension and allows meibum secreted by meibomian glands and tear lipids to spread with each blink. This creates a smooth optical surface for optimal vision. It also limits contamination from microorganisms and helps prevent tear evaporation.
The lipid layer is produced by meibomian glands, glands of Moll and Zeiss and epithelial cells. It is approximately 40nm to 60nm thick and can be further divided into different classes of lipids categorized into outer and inner layers. The outer lipid layer, known as the lipid-air interface, is composed of non-polar lipids such as cholesterols, triglycerides and free fatty acids. The inner layer, which makes up a smaller portion of the lipid-aqueous interface, is composed of ceramides, phospholipids and cerebrosides, known as polar lipids.6
The most common reason for lipid layer dysfunction is due to Meibomian gland dysfunction (MGD), which can be associated with a variety of systemic and ocular conditions, along with environmental factors and medications. External factors such as use of makeup eyeliner result in obstructive MGD, gland dropout, microtrauma and possible toxicity over time, resulting in progressive gland damage and then gland loss.7
Aqueous Layer
This layer is produced by the lacrimal gland mainly with contributions from goblet cells and accessory lacrimal glands. The main lacrimal gland is also responsible for reflex tearing. This layer is about 8mm thick and represents the bulk of the tear film. It’s important for lubrication, protection (by washing away contaminants and foreign bodies) and nourishing the avascular cornea. The aqueous layer includes proteins, immunoglobulins, glycoproteins, growth factors, vitamins and electrolytes important for ocular surface health.
Aqueous-deficient dry eye is associated with the lack of lacrimal gland secretions due to dysfunction or blockage of the glands, and is divided into Sjögren’s dry eye and non-Sjögren’s dry eye. Sjögren’s syndrome is an autoimmune condition associated with exocrine gland dysfunction, involving the salivary and lacrimal glands and results in dry eyes and dry mouth.8 It can be present solely as a primary diagnosis or secondary with other autoimmune associations such as rheumatoid arthritis or systemic lupus erythematosus.
In non-Sjögren’s dry eye, reduced aqueous can be due to several factors involving the lacrimal gland, which range from lacrimal duct obstruction or damage, conjunctival damage due to trauma or scarring (e.g., cicatricial pemphigoid, trachoma) and aging.
Any external factor that alters the composition of the aqueous will result in a compromised aqueous layer, turning into dry eye. Studies have shown that loss of growth factors associated with ocular surface disease or inflammation, increased electrolytes measured in tears (evaporation or exposure) and presence of proinflammatory cytokines will lead to disruption in the tear film.
When there is scant aqueous production, the ocular surface will have a reduced tear lake or high tear osmolarity. This is based on the amount of electrolytes in the aqueous layer, largely produced by the main and accessory lacrimal glands (Krause and Wolfring) from the cornea and conjunctiva.9 Tear hyperosmolarity is caused by reduced aqueous tear flow or increased evaporation of aqueous tear layer. With high tear osmolarity there is associated apoptosis of corneal, conjunctival and mucin-producing goblet cells.10 Therefore, patients with aqueous deficiency will have hyperosmolarity of the tear film, a reduced tear lake, Schirmer testing of less than 10mm and diffuse sodium fluorescein staining pattern.11
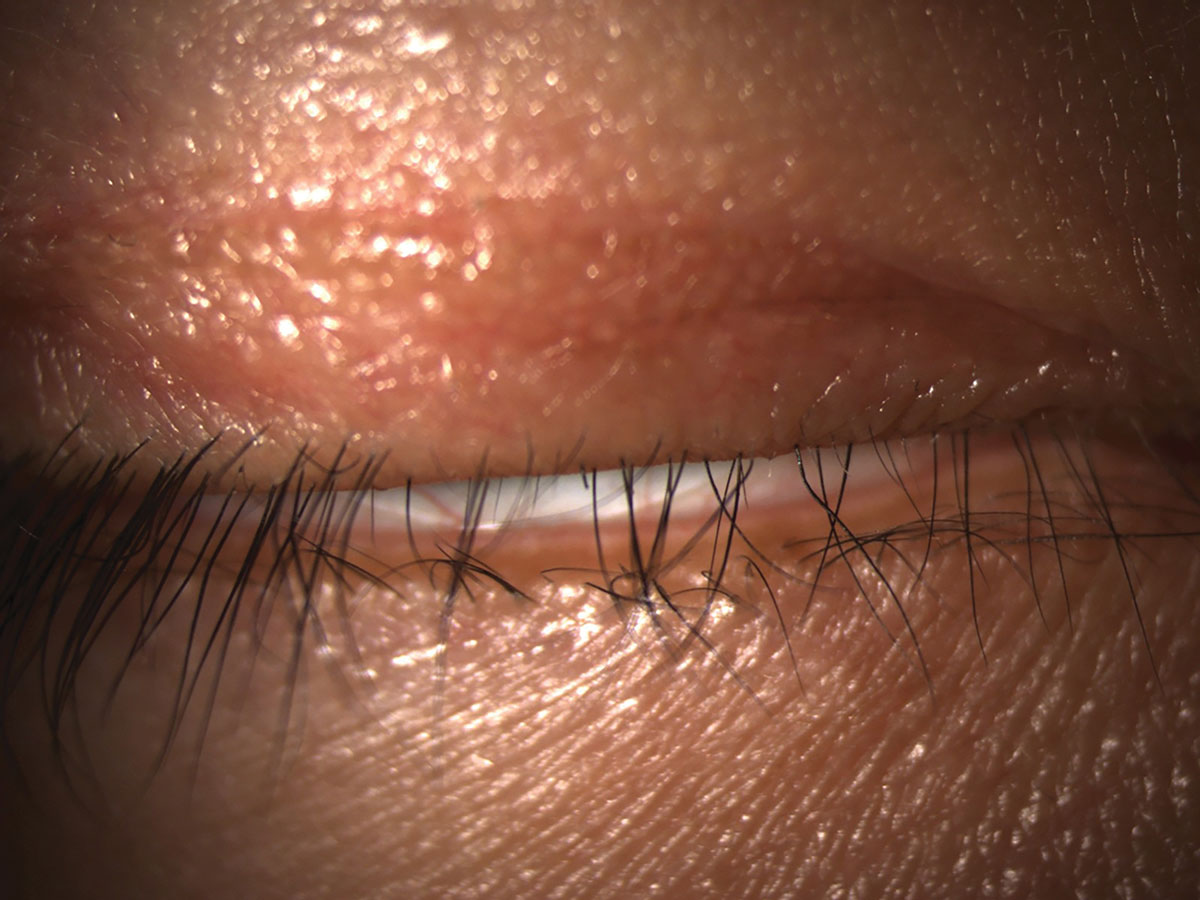 |
Lagophthalmos can cause inferior corneal exposure and contribute to DED. Click image to enlarge. |
Mucin Layer
This layer is important in stabilization of the tear film by making it hydrophillic and allowing the aqueous to spread over the ocular surface. Although it is suggested to be the thinnest layer of the three, the mucin layer plays a significant role in allowing the tear film to adhere to the surface by the gel-forming mucins and trapping and clearing contaminants and debris. Mucins are produced primarily by the goblet cells and apical cells of the cornea, conjunctiva and lacrimal gland.
Mucin deficiency is associated with conditions that have goblet cell damage, such as ocular cicatricial pemphigoid, Steven-Johnsons syndrome or other conjunctival disorders, and with vitamin A deficiency.12 Xerophthalmia is caused by severe vitamin A deficiency with the most common causes attributed to malnutrition or malabsorption due to poor diets, alcoholism, gastrointestinal, pancreatic and liver diseases.13
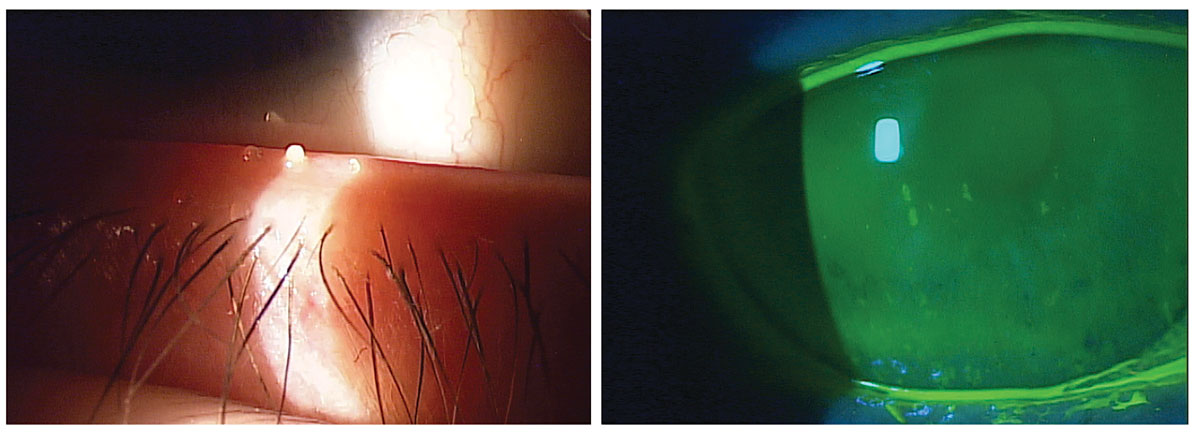
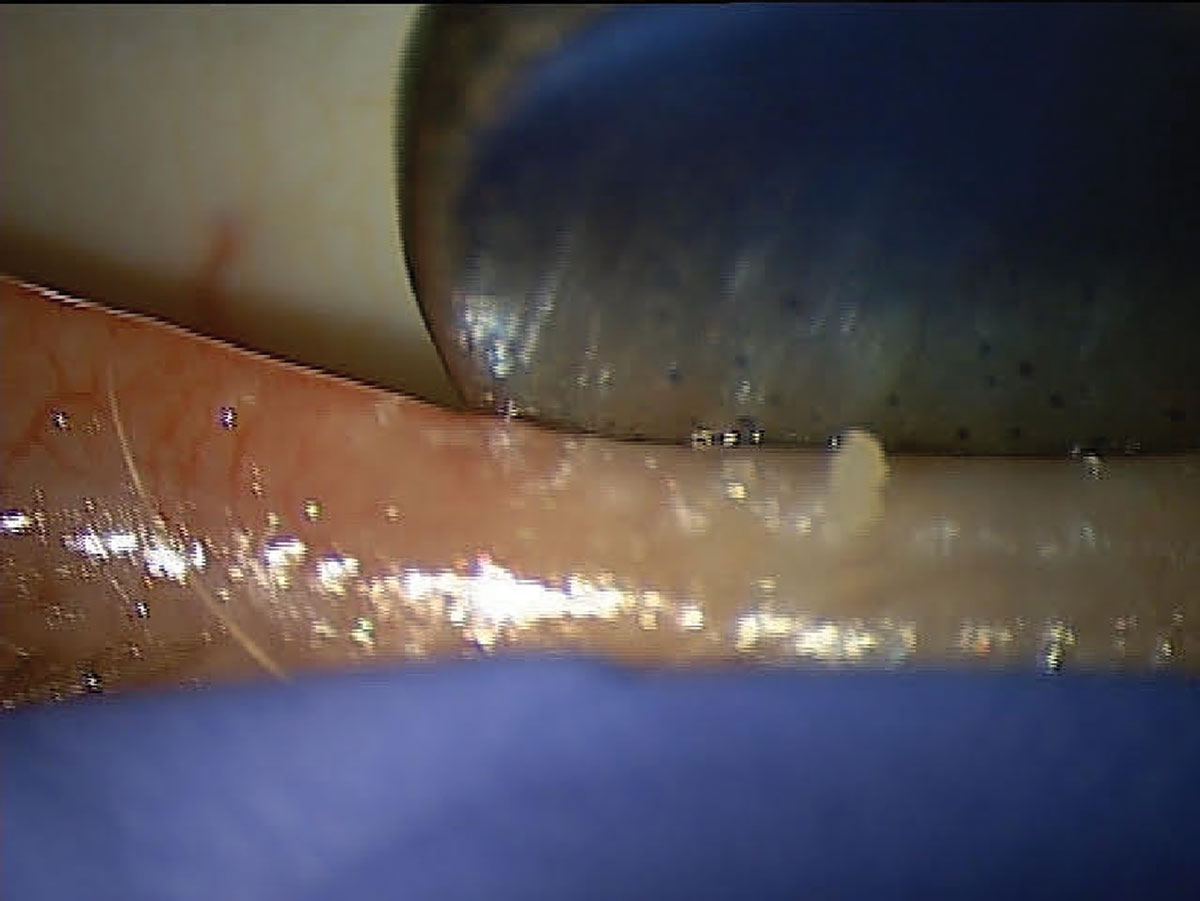 |
| A patient with DED associated with MGD showing poor MG expression. Click image to enlarge. |
Compromises to Tear Film
Each layer works independently but functions collectively as a unit to support the ocular surface and, similarly, can be damaged by conditions and external factors affecting all layers at once.14 These conditions include:
Aging. Increased aging results in tear film instability, decreased lacrimal secretion, increased evaporation rate, changes in lid apposition, conjunctival changes and change in tear film composition.15 Anatomical and physiological changes include atrophy or dysfunction of the lacrimal and meibomian glands, decreased nerve fiber density and long-term inflammation by infections or surgeries leading to decreased corneal sensation.
It is still unclear what exactly causes the changes in tear film composition, but some recent studies have shown tear film property differences between young and adult comparisons, including a decrease in antimicrobial lysozyme, lactoferrin and IgA proteins in the tears, with an increase in IgG, ceruloplasmin and other inflammatory interleukins and proteins in tears.16 Conjunctivochalasis has also been associated with causing instability of the tear film with aging. The exact etiology is not understood, but the tear film instability is likely due to the redundant conjunctival folds that decrease tear drainage and outflow and in turn release inflammatory MMPs from repeated friction with every blink.17
Infection. Another one of the tear film’s functions is to provide a protective barrier from external factors such as microorganisms and bacteria. The tear film has several antimicrobial proteins produced by the lacrimal gland and corneal and conjunctival epithelial cells.
Proteins such as lysozyme, lactoferrin, lipocalin and secretory immunoglobulin A produced by the lacrimal gland and found in the aqueous layer provide bacterial and antifungal protection, with lysozyme being the most abundant protein. Secretory immunoglobulin A is one of the primary antibodies in tears and functions to remove and clear pathogens from ocular surface.18 In aqueous-deficient dry eye, these proteins are reduced, leading to increased susceptibility of infections. When infections occur, there are changes to these antimicrobial enzymes resulting in decreased tear break up time, more tear instability, and more reflex tearing (due to increased aqueous secretion).
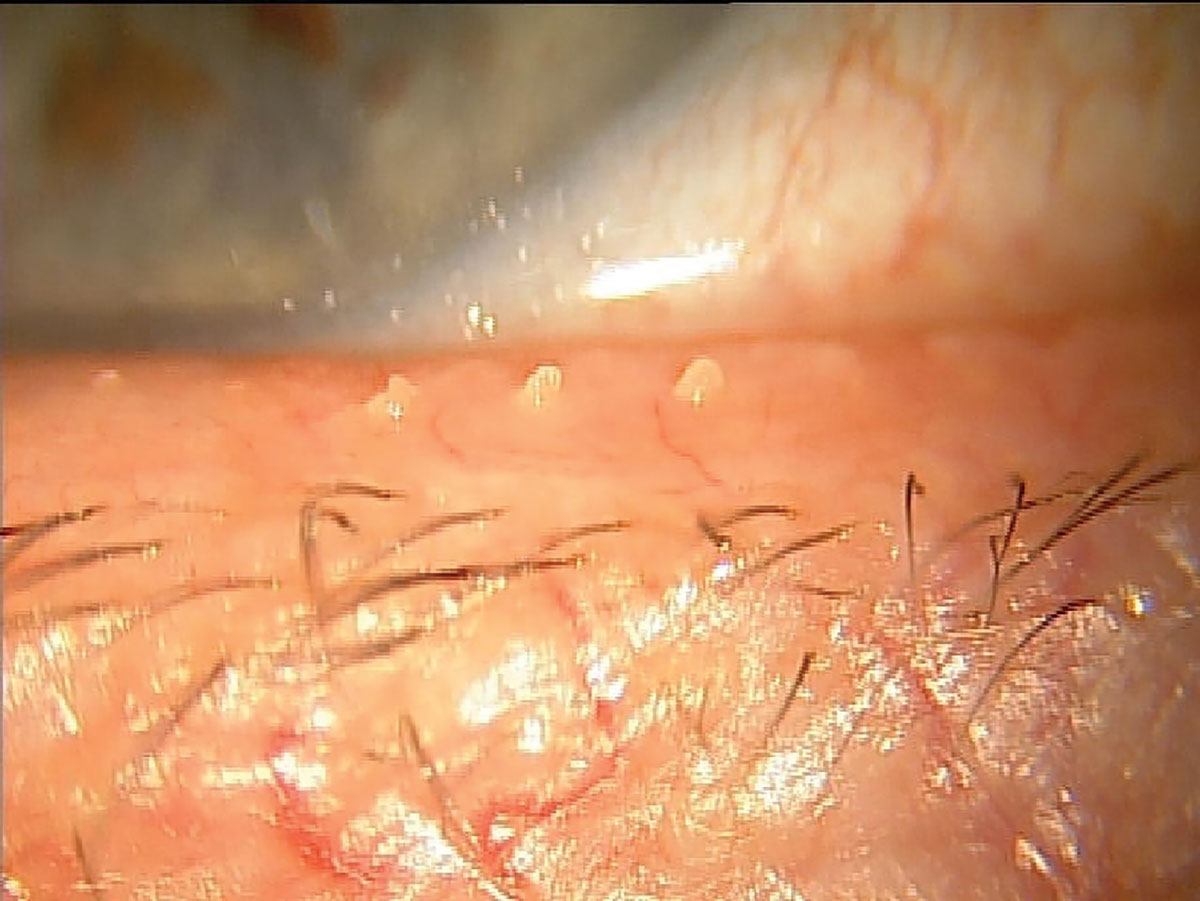 |
| Another MGD patient that likely suffers from chronic tear instability resulting from inadequate lipid release to the ocular surface. Click image to enlarge. |
Contact lens wear. There are several theories regarding changes to the tear film with contact lenses. Their use can promote increased tear evaporation, altered mucin production and reduced oxygen, as well as affect the concentration and lipids and proteins present in the tear film. Contact lenses are thought to disrupt the tear film by isolating the mucin layer behind the lens from the pre-lens lipid layer and impacting the natural congruity of the tear film layers.19 This disruption may lead to a reduced amount of antimicrobial proteins and make the ocular surface more susceptible to infections and inflammation.
It’s been noted that long-term wear of contact lenses, especially GP lenses, can decrease Müller’s muscle, leading to less or incomplete blinking which in turns leads to lipid layer deficiency and subsequent dry eye.20
Drugs. There are various medications that affect the tear film through different mechanisms, such as producing changes in the lacrimal gland, medications being secreted in the tears or systemically affecting overall secretions in the body. Over-the-counter painkillers, diuretics and chemotherapeutic agents can worsen dry eyes.
Many systemic medications that affect the tear film include anticholinergic drugs that have antimuscarinic effects. Antimuscarinics work by blocking muscarinic receptors from binding to the cholinergic receptors, which in turn lead to bronchodilation, mydriasis and inhibiting secretions, affecting the tear film. The cause of dry eye through this mechanism is decreased production of aqueous and mucin layer from the lacrimal and goblet glands respectively, leading to instability of tear film. Some of the most common medications associated with anticholinergic mechanisms that lead to decreased aqueous outflow are anxiolytics, antipsychotics, antidepressants, narcotics, and antihistamines and decongestants. Beta blockers in hypertensive medications also reduce aqueous production, leading to dry eye, while other medications such as topical isotretinoin or topical glaucoma eye drops can lead to meibomian gland dysfunction. Hormones, such androgens, TSH and estrogens, have been noted to influence lacrimal gland production or mucus secretion but require more studies to confirm.
Injury and/or ocular surgery. Changes in the tear film associated with ocular surgery include corneal nerve damage due to surgical incisions that reduce or disrupt tear production by damaging nerve fibers and corneal sensitivity and can lead to inflammation of the ocular surface. Light exposure and exposure to free radicals have also been questioned in causing damage to the corneal and conjunctival epithelial cells. (No significant data has confirmed this, but studies have noted decreased TBUT or Schirmer’s testing postoperatively that have either improved over time or remain slightly decreased).21 Blepharoplasty changes the position of the lids and can lead to tear film evaporation, while incisions cause surface irregularities or decreased mucin production resulting in compromised tear film.
Environmental influences. This includes low humidity, high altitude, pollution, wind, dust and allergens, all of which compromise the ocular surface. The quality of air and exposure to pollutants and allergens affects tear film stability and has been noted to be associated with increased inflammatory cytokines and MGD. Patients who live in cities with higher pollution levels or work in environments with increased dust, mold and smoke are more symptomatic to dry eyes due to increased TBUT by oxidative stress and inflammation. Similarly, patients living in certain regions or geographical locations with lower humidity or higher altitude are more susceptible to dry eye due to disruption of the ocular surface, resulting in increased tear film osmolarity and decreased TBUT.22
 |
Evaporative DED, a consequence of MGD (left), and aqueous-deficient (right), are the two primary types of dry eye. Click image to enlarge. |
Takeaways
Dry eye disease is a common, chronic condition. Since it is multifactorial in nature, an accurate diagnosis can be challenging but necessary for the most appropriate treatment. A stable tear film is important for a healthy ocular surface and, over the years, there have been several advances and insights into factors that affect ocular surface health.
As more studies are conducted, further understanding of the biochemistry and pathophysiology of the tear film will enhance diagnosis and treatment options to optimize homeostasis of the ocular surface for symptomatic patients. Because dry eye symptoms can be difficult to treat, educating patients on factors compromising the ocular surface can help with compliance as they incorporate lifestyle modifications to aid in maintaining a healthy, stable tear film.
Dr. Rojas is an instructor in optometric science (in ophthalmology) at Columbia University Medical Center. She received her undergraduate degree from the University of Central Florida and graduated from Nova Southeastern College of Optometry. She completed her optometric residency in ocular disease at SUNY College of Optometry. She specializes in medically necessary contact lens fittings and dry eye, and comanages refractive, corneal and ocular disease. She has no financial disclosures.
1. Lemp MA. Report of the National Eye Institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21(4):221-32. 2. Craig JP, Nichols KK, Akpek EK. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276-83. 3. Nelson JD, Craig JP, EK Akpek, et al. TFOS DEWS II introduction. Ocul Surf. 2017;15(3):269-75. 4. Davidson HJ, Kuonen VJ. The tear film and ocular mucins. Vet Ophthalmol. 2004;7(2):71-7. 5. Kawashima, M, Tsubota, K. Tear lipid layer deficiency associated with incomplete blinking: a case report. BMC Ophthalmol. 2013;13:34. 6. Masoudi S. Biochemistry of human tear film: a review. Exp Eye Res. 2022;220:109101. 7. Prabhasawat P, Chirapapaisan, C, Chitkornkijsin C, et al. Eyeliner induces tear film instability and meibomian gland dysfunction. Cornea. 2020;39(4):473-8. 8. Hu S, Vissink A, Arellano M, et al. Identification of autoantibody biomarkers for primary Sjögren’s syndrome using protein microarrays. Proteomics. 2011;11(8):1499-507. 9. Stahl U, Willcox M, Stapleton F. Osmolality and tear film dynamics. Clin Exp Optom. 2012;95(1):3-11. 10. Baudouin C, Aragona P, Messmer EM, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN Group Meeting. Ocul Surf. 2013;11(4):246-58. 11. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27 Suppl 1:3-47. 12. Hodges RR, Dartt DA. Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp Eye Res. 2013;117:62-78. 13. Xerophthalmia. EyeWiki. www.eyewiki.aao.org/xerophthalmia. May 16, 2022. Accessed December 13, 2022. 14. Hao R, Zhang M, Zhao L, et al. Impact of air pollution on the ocular surface and tear cytokine levels: a multicenter prospective cohort study. Front Med (Lausanne). 2022;9:909330. 15. Nättinen J, Antti J, Ulla A, et al. Age-associated changes in human tear proteome. Clin Proteomics. 2019;16:11. 16. McGill JI, Liakos GM, Goulding N, Seal DV. Normal tear protein profles and age-related changes. Br J Ophthalmol. 1984;68:316-20. 17. Yvon C, Patel BC, Malhotra R. Conjunctivochalasis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. 18. Mann A, Campbell D, Mirza Z, et al. Clinical and biochemical analysis of the aging tear film. Br J Ophthalmol. 2020;104:1028-32. 19. Rohit A, Willcox M, Stapleton F. Tear lipid layer and contact lens comfort: a review. Eye Contact Lens. 2013;39(3):247-53. 20. Bleyen I, Hiemstra CA, Devogelaere T, et al. Not only hard contact lens wear but also soft contact lens wear may be associated with blepharoptosis. Can J Ophthalmol. 2011;46(4):333-6. 21. Mikalauskiene L, Grzybowski A, Zemaitiene R. Ocular surface changes associated with ophthalmic Surgery. J Clin Med. 2021;1210(8):1642. 22. Willmann G, Schatz A, Fischer MD, et al. Exposure to high altitude alters tear fi lm osmolarity and breakup time. High Alt Med Biol. 2014;15(2):203-7. |


