 |
We’ve all had patients who wear their contact lenses for extended hours. In this case, my patient presented having worn his lenses for approximately 8,736 hours straight! Still, he noted good comfort and vision from these lenses, though some deposits were becoming noticeable and made him seek care. We ultimately chose to refit him into a GP lens design to optimize corneal health.
Case Presentation
 |
|
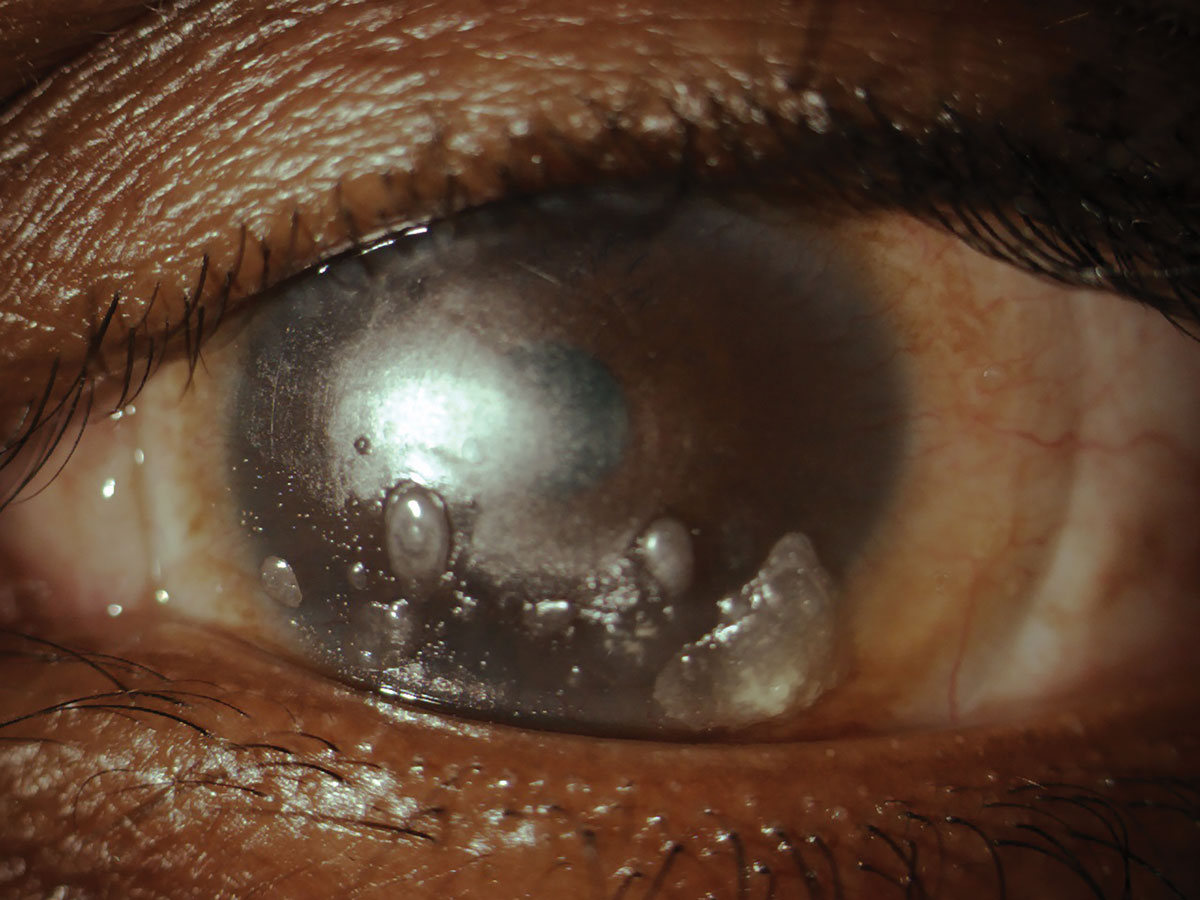
Fig. 1. Heavily deposited soft toric contact lens. Click image to enlarge. |
A 43-year-old African American male presented to the clinic for a contact lens fitting OU. He had just seen our primary care service six days earlier and was prescribed spectacles which were on order. He stated he wore “hard contacts” that he was prescribed approximately one year prior at an outside practice. He reported that the lenses were causing itching symptoms. He admitted to wearing the lenses 24 hours a day, seven days a week—removing them just once per week to clean them. Cleaning consisted of lens removal and rubbing with an unknown lens solution followed by placing the lenses inside the case overnight. The lenses had never been replaced since they were obtained.
He had noticed deposits developing on the lenses over the last four to five months. He did not feel like his best-corrected vision was reduced but did complain of glare while driving at night. He did not currently have backup glasses. Entering VA was 20/50 OD and 20/50 OS with the patient’s habitual lenses. Pupils, extraocular muscles and confrontation visual fields were all within normal limits. The patient was oriented to time, place and person and his mood/affect was normal.
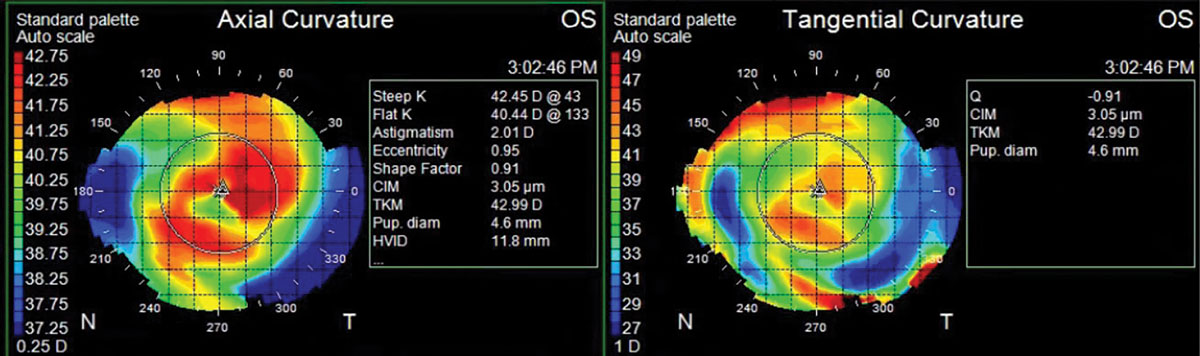
Contact lens assessment showed tight-fitting, well-centered, soft toric lenses with heavy central and inferior deposits and no movement OU (Figure 1). There was no over-refraction that improved vision in either eye. The lenses did not appear rotated. After lens removal, manifest refraction results were -10.25 -1.50 x 120 OD and -13.00 -3.00 x 095 OS with no improvement in best-corrected visual acuity (BCVA) from entering. Corneal topography revealed simulated keratometry values of 42.35/41.01@057 OD and 42.45/40.44@133 OS. Both maps showed steepening due to overwear of the steep-fitting lenses (Figure 2).
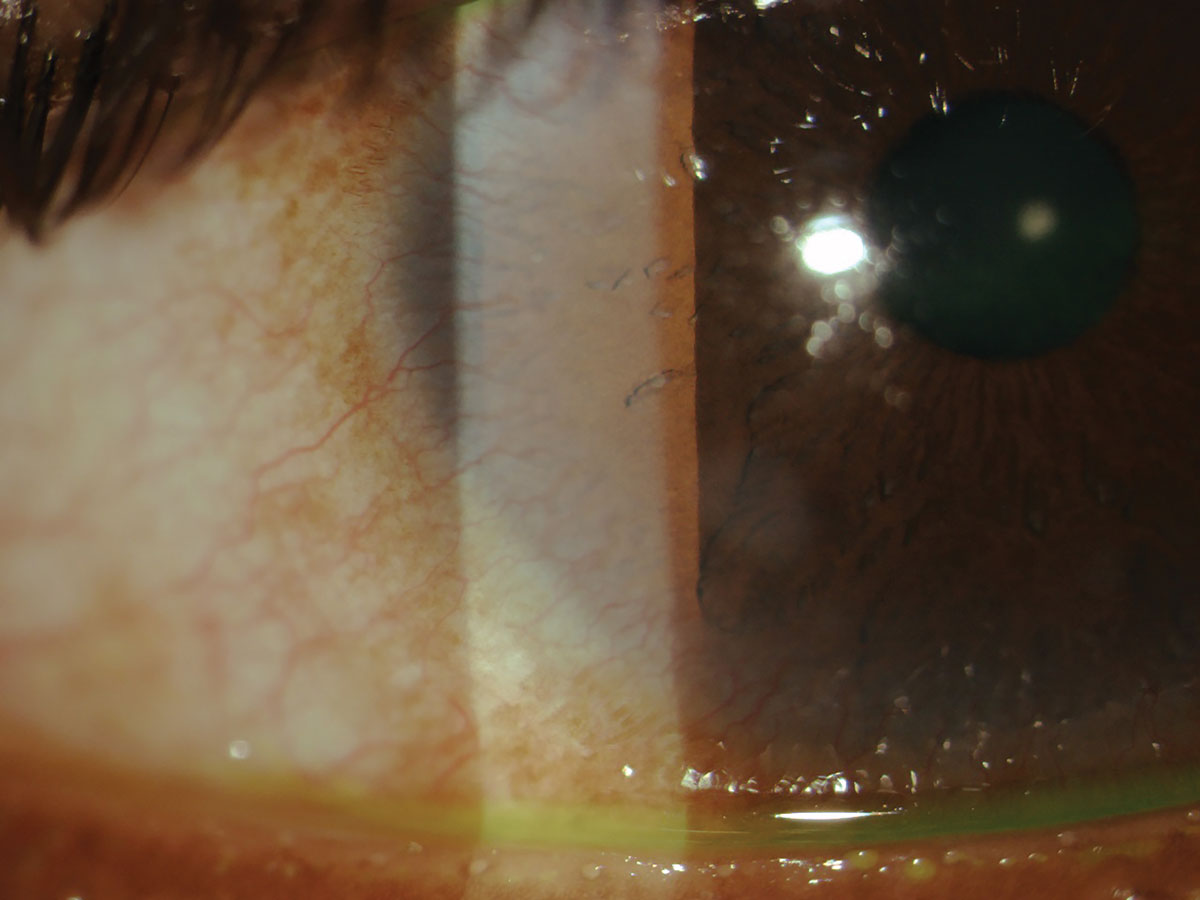
Upon further slit lamp examination, the lids and lashes were clear. Everting the upper and lower lids showed large palpebral conjunctival papillae and 3+ injection on both the lids in each eye. Corneal evaluation revealed peripheral corneal opacities OD>OS and central punctate sodium fluorescein staining OU. There was also extensive corneal neovascularization present at the limbus, 360º in each eye and greater inferiorly, presumably due to the patient’s long history of soft toric lens wear and prior contact lens overwear (Figure 3).
The patient was diagnosed with giant papillary conjunctivitis OU, worse on the upper lid, from presumed contact lens overwear. He was started on prednisolone acetate 1.0% ophthalmic solution QID OU and advised to follow-up in two weeks for evaluation and intraocular pressure check. The corneal neovascularization was photodocumented for comparison at future visits.
New spherical corneal GP lenses were fit in a low mass ophthalmic design and a high Dk material. The patient was advised to discontinue wear of the habitual, heavily deposited soft toric lenses as soon as his new spectacles arrive to allow for the corneal warpage to resolve. Of note, it takes approximately three weeks for warpage to resolve from soft contact lens wear.1 In contrast, warpage from GP lens wear can take up to five months to resolve.2
 |
|
Fig. 2. Topography maps showing changes to tangential curvature consistent with a tight-fitting lens and associated corneal warpage. Note that the eccentricity value is abnormally high. Click image to enlarge. |
Lens Refit
New lenses were designed for the patient. Due to the degenerative myopia and low amount of corneal cylinder (<3.00D), we chose the Thinsite2 (Art Optical) spherical corneal lens design OU, as it has features particularly useful in patients with high ametropia.3 The final parameters of the Thinsite2 lens designed was OD 7.9/9.5/-11.50DS and OS 7.9/9.5/-12.75DS in Boston XO fluorosilicone acrylate material. The lenses were ordered in green material OD and blue material OS, so the patient did not mix up the lenses. The lenses were ordered with a slightly larger 9.5mm diameter to further improve initial comfort.
The fit showed a well-centered lens with central alignment, mid-peripheral bearing and a minimally acceptable amount of edge lift with good centration and a lid attached fit (Figure 4). The lens was ordered, dispensed and followed-up over the new few months and the patient wore it successfully for the next year.
A Good Option
The Thinsite2 lens design was designed to fill the void left by the success of the low Dk Polycon II lens design—a low mass corneal lens that was able to retain rigidity and strength.4,5 This specialized lens design features a posterior surface and an anterior surface with a spherical central optical correction zone, an aspheric intermediate zone and a peripheral zone.4 The central optical zone of the anterior surface has a larger diameter than the central optical zone of the posterior surface. Also, the intermediate zone of the anterior surface has a larger diameter than the intermediate zone of the posterior surface.4 These features are beneficial when applied to GP lenses made using high Dk materials.
Patients wearing corneal GP lenses often experience lens awareness, which is worsened by a lens with any significant amount of mass. Eyecare practitioners have sought to reduce the center thickness of these lenses to reduce their mass and increase their oxygen permeability; however, this can weaken the lens structure. These weaker lenses, while healthier for the patient, carry an increased risk of breakage, warpage and flexure.4
Thinsite2 design’s reduced lens mass allows for improved centration of the optical zone when compared with similar prescriptions in standard lens designs.3 This ultra-thin design maximizes oxygen transmission for optimal corneal health—particularly important for patients with high ametropia. The lens is also manufactured with a junctionless aspheric front and back surface, which reduces lens-lid interaction as well as the patient’s lens awareness. This eases patient adaptation, making this lens design a good option for any new GP lens fit or refit.3 The added comfort was useful here as the patient was accustomed to the comfort of a soft toric lens. Despite a thin profile, the Thinsite2 lens’s unique design is able to resist flexure and give an improved lens-to-cornea fitting relationship for higher prescriptions.3
 |
|
Fig. 3. Corneal neovascularization from overwear of a high-powered soft toric lens. Click image to enlarge. |
Slit-lamp examination revealed mild vessel encroachment at the limbus nasal and temporal in each eye. There was no fluorescein staining in either eye after lens removal. Today’s refraction was relatively stable to previous visits at OD -19.75DS with VA 20/30 and OS -17.25 -2.50 x 165 with VA 20/25. The dilated fundus evaluation revealed stable white-without-pressure and lattice degeneration OU.
We re-educated the patient on the importance of nightly lens removal and cleaning procedures. He was advised to replace his lenses as the deposits are extensive and were not removed with our in-office cleaning procedures. We also discussed the limitations on the patient’s vision. Though he stated he was happy with his entering acuity, it was reduced to 20/70 in the poorer seeing eye. We also discussed the importance of having backup glasses in order to limit lens wear time.
1. Wilson SE, Lin DT, Klyce SD, et al. Topographic changes in contact lens-induced corneal warpage. Ophthalmology. 1990;97(6):734-44. 2. Rayess Y, Arej N, Massih YA, et al. Influence of soft contact lens material on corneal warpage: prevalence and time to resolution. Can J Ophthalmol. 2018;53(2):135-8. 3. Thinsite2 product fitting information. Art Optical. www.artoptical.com/products/thinsite2. Accessed July 17, 2022. 4. Hodur NR, Caroline PJ, inventors; Art Optical Contact Lens Inc, assignee. Low-mass ophthalmic lens. US Patent No. 6,325,509. December 4, 2001. 5. Hodur NR, Caroline PJ, inventors; Art Optical Contact Lens Inc, assignee. Low-mass ophthalmic lens. US Patent No. 6,520,637. February 18, 2003. |


