The endothelium—the posterior facing and thinnest layer of the cornea—is often taken for granted. This simple layer plays a critical role in maintaining transparency of the “window” of the eye, allowing visualization not only of the anterior ocular structures but also the posterior segment. This fragile structure is comprised of a monolayer of six-sided cells, void of replication capabilities and tasked with maintaining structural hydration. Understanding the pathophysiology of abnormal function is vital for clinicians to develop a refined treatment and management plan when faced with a cloudy cornea.
The Norm
Overall, the barrier to external invaders is maintained by the corneal epithelium; maintenance of thickness and shape for refractive power is provided via the stroma, and hydration and nutrition are the responsibility of the endothelium.1 Corneal nutrients, save oxygen, are predominantly obtained from the aqueous humor via passive diffusion and active transport on the apical and basolateral flanks of the endothelium.2,3 The endothelium has a two-part job in its role of maintaining hydration and nutrition with an active “pump” and a passive “leak” component.4 The resultant hydrostatic pressure of the aqueous and the oncotic pressure of the cornea maintains tissue deturgescence.5
Endothelial cell patterns are specifically designed to facilitate their function and often mimic that of “foam-like” material.6 Endothelial hexagonality falls in line with von Neumann-Mullins law, which indicates that cells with greater than six sides grow and cells with fewer shrink—allowing hexagonal cells to maintain equilibrium.6 Cell growth in adults demonstrates a classic linear pattern with mean cell size (MCS) increasing proportionally with age and a corresponding decrease in endothelial cell density (ECD).6 Moreover, there are slightly more smaller cells in the periphery compared with centrally, thought to occur due to finite corneal space.6
The classic hexagonality that is visualized when considering corneal endothelial cells (ECs) only holds true for their apical surface, which is in contact with the aqueous humor; the basal surface has been modeled to be irregular in nature.3 The apical, hexagonal shape is maintained by tight junctions and a sub-membranous network of actomyosin along the lateral borders where the ion pumps reside.3
Senescence
Aging affects all parts of the visual system. However, it affects ECs particularly harshly, as they cannot rely on regeneration to maintain function and are subject to apoptosis over their lifetime.5,7 The average adult holds an ECD of approximately 2,500 to 3,000 cells per mm2; however, corneal clarity can be maintained at a critical mass of 400 to 700 cells per mm2 in individuals with average intraocular pressure (IOP).8
Due to this natural and ongoing deterioration of EC numbers, the remaining cells often morph via centripetal migration and stretching in order to compensate for lost comrades.9 In the absence of mitosis in vivo, changes in MCS (polymegethism) and cell shape (pleomorphism) are direct consequences of the reduction in ECD—namely, disruption of the pump-leak process required to maintain tissue transparency.7,10 There is evidence of this change in uniformity when reviewing the coefficient of variation (CV) of mean cell area—a measure of polymegethism—and the proportion of hexagonal cells—a measure of pleomorphism. With time, the former increases from 0.22 to 0.29 and the latter decreases from 75% to 60%.11
Genetics
The most commonly encountered endothelial corneal dystrophies include Fuchs’ dystrophy, posterior polymorphous dystrophy (PPD) and congenital hereditary endothelial dystrophy (CHED). Though the three conditions have varying genetic bases and modes of inheritance, all lead to a mutation’s resulting errors in transcription factors, structural components of the stroma and Descemet’s membrane, or cell transport proteins.12 Regardless of genetic cause, each dystrophy can halt the pump-leak process of the corneal endothelium, leading to edema, increased scattering of light, scarring and loss of vision.
Fuchs’ is the most common corneal endothelial dystrophy and includes two distinct genetic variations: early-onset Fuchs’ and the more common late-onset Fuchs’, both marked by the presence of guttae greatest centrally.13 Early-onset Fuchs’ includes a mutation of the COL8A2 gene thought to have autosomal dominant inheritance and lead to changes in a component of Descemet’s membrane.12,14
Late-onset Fuchs’, however, reveals high genetic heterogeneity and has been associated with several genes, including FECD2 (13pter-q12), FECD3, TCF4, FECD4, SLC4A11, FECD5 (5qdd-35), FECD6, ZEB1, FECD7 (9q24-22), FECD8, AGBL1, FECD, DMPK and TCF4 intronic repeat sequence. This type of Fuchs’ displays mixed effects, like disrupted functions for Descemet’s membrane, transcription factors, ion transport, glutamate decarboxylase and protein kinase.12 Areas between guttae demonstrate increased CV and mean cell area.12 Unlike with senescence, enlarged ECs are noted to secrete excess basement membrane in the form of banded collagen, further exasperating the pump-leak dysfunction.13
 |
|
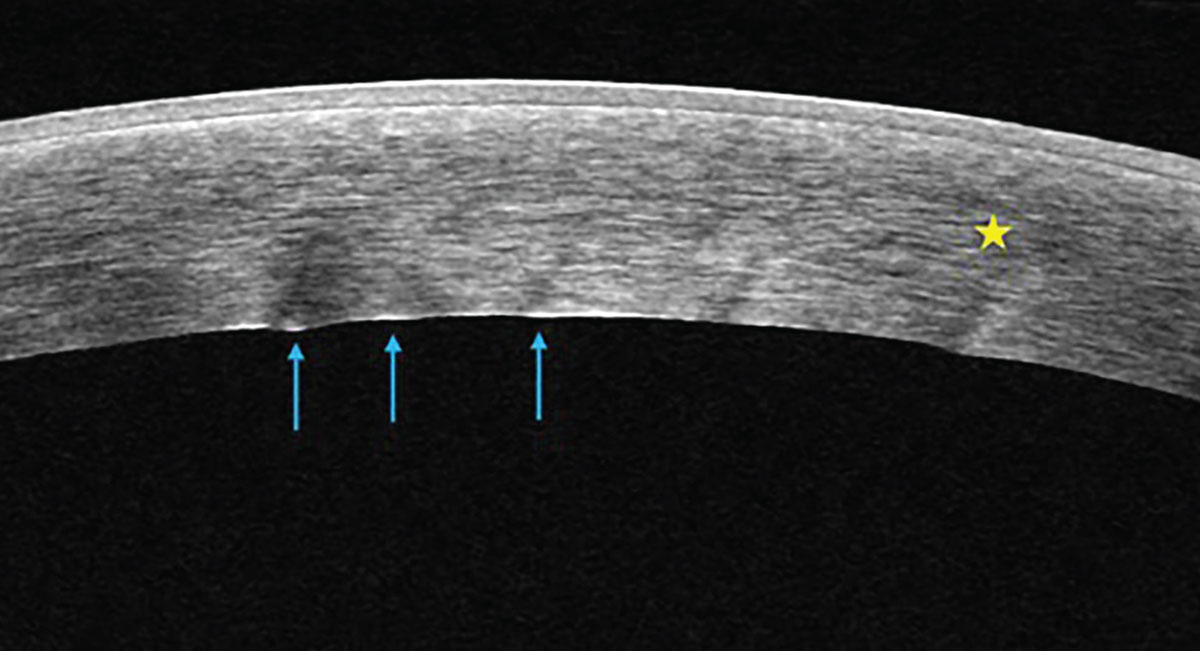
Anterior segment OCT of a cornea with microcystic stromal edema (yellow star) and endothelial folds (blue arrows) secondary to endothelial pump dysfunction from herpetic disease. Click image to enlarge. |
PPD includes epithelium-like characteristics of the endothelium, but with less severe clinical signs and symptoms. However, there is an increased possibility for extension of the trabecular meshwork and a resultant rise in IOP.13 Mutations in OVOL2 (PPCD1), ZEB1 (PPCD3) and GRHL1 (PPCD4) and autosomal dominant inheritance are known to be associated with PPD.12 Unsurprisingly, these genes are key regulators of differentiation, and variance leads to changes in cell type, either from epithelial to mesenchymal or vice-versa.12
CHED, the endothelial dystrophy present from birth, has both a dominant and recessive form and is associated with OVOL2 and SLC4A11, respectively.12 There is significant stromal haze in both forms and a strong association with childhood glaucoma.12 The autosomal recessive form presents with a milky cornea in the first few weeks of life, along with diffuse opacity, a ground-glass appearance, increased corneal thickness, lack of ECs and homogenous thickening of Descemet’s membrane.12 Alternately, the autosomal dominant form develops opacity after the first year of life and appears with an absence of an endothelial mosaic.12
Trauma
The corneal endothelium responds to injury in three stages:
- Migration of adjacent ECs to fill the wound
- Re-establishment of tight junctions
- Remodeling of ECs into a regular hexagonal shape4
The initial stage is immediate, and the latter two stages can take up to several months.4 The corneal endothelium is remarkably resilient to injury—whether that be in the form of trauma or ocular surgery. Its tenacity is due to several factors, including increased peripheral endothelial cell number ready for migration, ability of ECs to form new tight junctions to maintain function, an increase in endothelial Na+/K+ ATPase pump sites under stress, homeostatic concentrate of Na+ in the aqueous humor allowing the osmotic gradient and the ability of ECs to shift their metabolism of glucose to the hexose monophosphate shunt for NADPH and membrane repair.15
The impact of various intraocular surgeries on the corneal endothelium and resultant bullous keratopathy have been studied in order to establish best practices, with cataract extraction having the most data. Manual small-incision cataract surgery with posterior chamber intraocular lens implantation has been shown to cause 15.8% endothelial cell loss.16 With or without intraocular lens implantation, ECD has been demonstrated to continue to decrease at an annual rate of 2.5% for at least 10 years after initial decline.17
Post-cataract surgery endothelial cell loss has revealed to be amplified in diabetic patients compared with unaffected individuals.18 Furthermore, phacoemulsification in the context of a decreased endothelium to iris distance has been associated with increased rate of EC loss.19 Surgeons often avoid endothelial compromise by remaining cognizant of chamber depth, cataract density, phaco time, surgery time, ultrasound power, intraocular lens contact, instrument-related trauma, incision size, incision location, irrigation solution turbulence, intraocular lens type and viscoelastic substance.18,20 Additionally, hydrogen in the form of H2 dissolved in ocular irrigation solution allows protection of ECs from phacoemulsification-induced oxidative stress and damage.21
 |
|
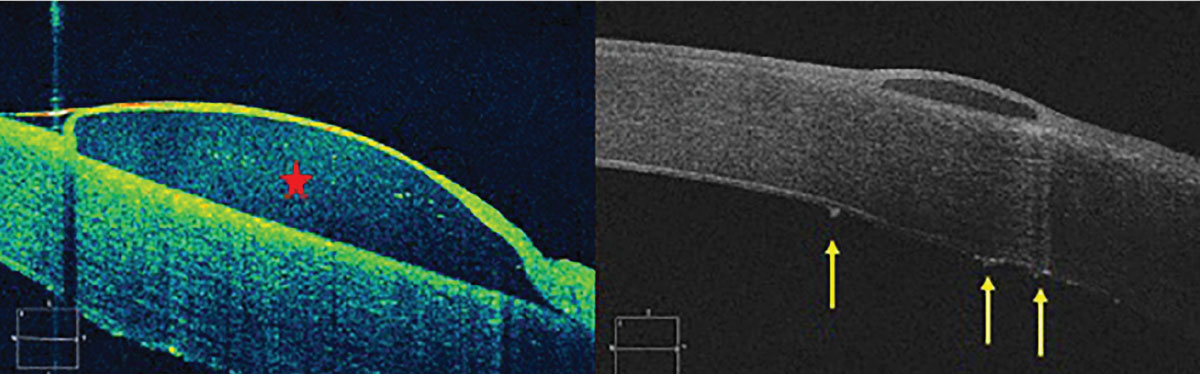
Anterior segment OCT of a corneal bullae (red star) in a patient with uncontrolled Fuchs’ endothelial dystrophy leading to stromal edema and ultimately bullous keratopathy. Left photo shows initial presentation and right photo is near resolution with large endothelial guttae visible (yellow arrows). Click image to enlarge. |
ECD loss is evident in suprachoroidal microinvasive glaucoma surgery (MIGS), Schlemm’s canal implantable devices, Schlemm’s canal procedures without implantable devices, trabeculectomy and aqueous shunt surgery.22 Combined glaucoma MIGS involving long-term implants do not show more endothelial decompensation than glaucoma filtration surgeries alone.22
There has been evidence of corneal decompensation with laser iridotomy and Nd:YAG laser with proposed mechanisms for direct focal injury, thermal damage, mechanical shock waves, iris pigment dispersion, transient rise in IOP, inflammation, turbulent aqueous flow, time-dependent shear stress on ECs, chronic breakdown of the blood-aqueous barrier and damage from bubbles that settle on the endothelium.23 The need for Nd:YAG laser capsulotomy within one year of cataract extraction has been postulated as the mechanism for post-op ECD decline.24 Endothelial health should also be considered in posterior segment procedures as ECD exhibits a decrease in retinal photocoagulation with an indirect ophthalmoscopy contact lens.25
Contact Lenses
Hypoxic stress can cause endothelial changes in cell morphology, including polymegethism, pleomorphism and alteration of microanatomy that can be linked to contact lens wear.13,26,27 Though not as common, hard and soft polymethylmethacrylate lenses were noted to cause the most significant changes in ECD.27
There is less evidence that ECD or central corneal thickness is affected by chronic contact lens use of newer materials.26 Endothelial intercellular pH changes due to hypoxia-induced CO2 increase, lactate buildup, reduced availability of anterior chamber oxygen and endothelial bleb (small black spots appearing as disruptions on the endothelium) formation may all be culprits of endotheliopathy in contact lens users.28 What’s more, patients with corneal grafts can be at a higher risk for further endothelial compromise, and those fit in specialty contact lenses should be closely monitored.
Disease Sequelae
Incidence of glaucoma has been linked to changes in ECD due to the suggested mechanism of increased IOP accelerating cell loss.29 When looking at cell density in patients with pseudoexfoliation post-cataract surgery, those with glaucomatous damage demonstrated a decreased ECD when compared with those with the syndrome.30
Carbonic anhydrase has an important function in the pump-leak process of the endothelium, thus carbonic anhydrase inhibitors (CAIs) that can cause pump dysfunction are a natural culprit to consider. Endothelial pump deceleration, acidification of the ECs and interference with intercellular pH regulation have been noted when CAIs are applied directly to ECs in vitro.31 Topical and oral CAIs—such as dorzolamide or brinzolamide—have been anecdotally linked to increased corneal thickness and decreased ECD; however, this has not been proven to be clinically significant in studies on patients with normal baseline ECD.32,33
 |
|
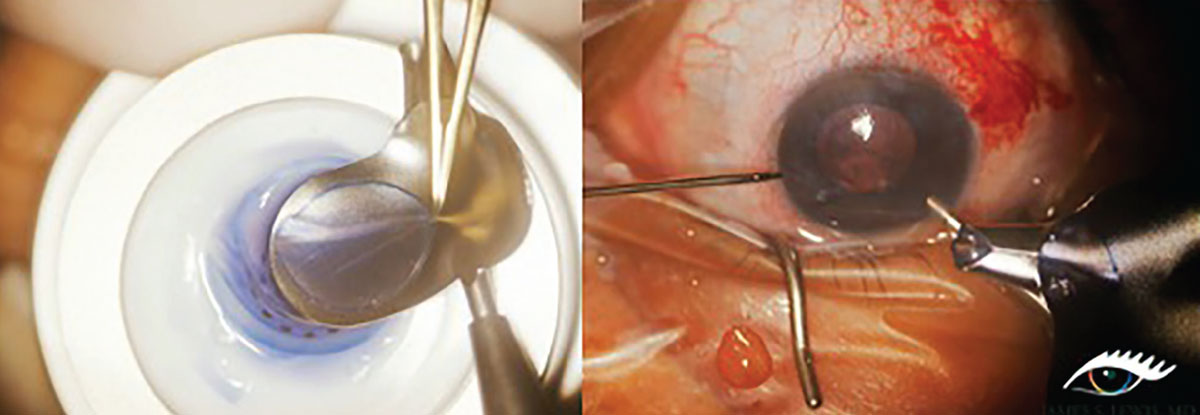
Left: A 9mm donor graft is loaded in preparation for DSEK procedure in patient with severe endothelial decompromise. Right: Donor tissue is folded up like a taco with endothelium side up in order to insert through a temporal incision. Photo: James Lewis, MD. Click image to enlarge. |
In individuals with pre-existing endothelial dysfunction, the use of dorzolamide has been linked to a 12µm thickness increase.34 Endothelial toxicity to benzalkonium chloride or other preservatives, rather than the active medication, has been hypothesized to cause breakdown of the apical barrier’s functioning, but changes are thought to be dose- and time-dependent.35,36 Finally, there is some suggestion that topical prostaglandin analogs, beta-blockers, alpha agonists, CAIs and miotics used for glaucoma management may affect physiological endothelial function via changes in Ca2+ mobility.37
Changes in MCS, cell shape, increased rate of endothelial cell death and corneal edema—similar to that seen in Fuchs’—have all been demonstrated in patients with diabetes mellitus (DM).38 One study found that there was an 11% reduction in ECD in DM Type 1 vs. a 5% reduction in DM Type 2, while displaying a progressive decline in ECD with disease duration and higher HbA1c.37,39,40 Evidence for cell mitochondrial swelling, metabolic dysfunction, decreased Na+/K+ ATPase function, poor tight junction formation and changes to the extracellular matrix have been linked as potential causes.37
Though there is confounding literature, most studies suggest that diabetes accelerates endothelial cell dropout and is associated with decreased central ECD and increased CV likely secondary to metabolic changes causing endothelial instability.41 Even further, chronic kidney disease in patients with or without DM can be linked to vulnerability in decompensation due to polymegethism and pleomorphism.42
Secondary inflammation of the endothelium—called endotheliitis—has been connected to viruses of the herpes family. Characteristic findings include keratic precipitates, anterior chamber inflammation, endothelial dysfunction and resultant corneal edema. The inflammation is classified into four discrete types: linear, sectoral, disciform and diffuse.43 It has been postulated that this is likely an anterior chamber–associated immune deviation disease modulated by viral antigens.44 This gives credibility to the idea that systemic viral pathogens can cause an inflammatory response in ocular structures; in fact, recent evidence for patients testing positive for COVID-19 suggests that endothelial changes during active infection could help understand the systemic effects of the disease.45
Takeaways
The endothelium warrants further attention than its 5µm thickness initially implies, as it plays a key role in corneal clarity. Considerations go well beyond initial understanding and require continuous re-evaluation. Astute optometrists should evaluate the structure carefully in order to unearth subtle changes that can later lead to deleterious effects. Additionally, linking ocular pathology to systemic considerations is vital in holistic care of our patients.
Dr. Minhas currently serves as the Associate Dean of Accelerated Programs at Pennsylvania College of Optometry (PCO) at Salus University. She oversees the three-year Accelerated Scholars Program and the Advanced Placement Optometry Degree Program in this capacity. She works closely with students in the Traditional and Scholars programs and in the residency program at PCO in clinic, lecture, lab and grand rounds. She has no financial interests to disclose.
1. Rosado-Adames N, Afshari NA. “Corneal endothelium.” Yannoff M. Ophthalmology, 4th edition. Saunders. 2013;264-8. 2. Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res. 2012;95(1):2-7. 3. He Z, Forest F, Gain P, et al. 3D map of the human corneal endothelial cell. Sci Rep. 2016;6(29047). 4. DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37(3):588-98. 5. Abib FC, Hida RY, dos Santos RM. Corneal endothelium: histology, physiology and in-vivo examination with specular microscope. JSM Ophthalmol. 2017;5(4):1063. 6. Wörner CH, Olguín A, Ruíz-García JL, Garzón-Jiménez N. Cell pattern in adult human corneal endothelium. PLoS One. 2011;6(5):e19483. 7. Claerhout I, Beele H, Kestelyn P. Graft failure: I. Endothelial cell loss. Int Ophthalmol. 2008;28(3):165-73. 8. Murphy C, Alvarado K, Juster R, Maglio M. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest Ophthalmol Vis Sci. 1984;25(3):312-22. 9. Polse KA, Brand RJ, Cohen SR, Guillon M. Hypoxic effects on corneal morphology and function. Invest Ophthalmol Vis Sci. 1990;31(8):1542-54. 10. Faye PA, Poumeaud F, Chazelas P, et al. Focus on cell therapy to treat corneal endothelial diseases. Exp Eye Res. 2021;204:108462. 11. Yee RW, Matsuda M, Schultz RO, and Edelhauser HF. Changes in the normal corneal endothelial cell pattern as a function of age. Curr Eye Res. 1985;4(6):671-8. 12. Kannabiran C, Chaurasia S, Ramappa M, Mootha VV. Update on the genetics of corneal endothelial dystrophies. Indian J Ophthalmol. 2022;70(7):2239-48. 13. Bourne WM. Biology of the corneal endothelium in health and disease. Eye (Lond). 2003;17(8):912-8. 14. Sacchetti M, Macchi I, Tiezzi A, et al. Pathophysiology of corneal dystrophies: from cellular genetic alteration to clinical findings. J Cell Physiol. 2016;231(2):261-9. 15. Edelhauser HF. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea. 2000;19(3):263-73. 16. Singh M, Mishra D, Sinha BP, Anand A, Singhal S. Corneal endothelial protection during manual small-incision cataract surgery: a narrative review. Indian J Ophthalmol. 2022;70(11):3791-6. 17. Bourne WM, Nelson LR, Hodge DO. Continued endothelial cell loss ten years after lens implantation. Ophthalmology. 1994;101(6):1014-22. 18. He X, Diakonis VF, Alavi Y, et al. Endothelial cell loss in diabetic and nondiabetic eyes after cataract surgery. Cornea. 2017;36(8):948-51. 19. Hwang HB, Lyu B, Yim HB, Lee NY. Endothelial cell loss after phacoemulsification according to different anterior chamber depths. J Ophthalmol. 2015;2015:210716. 20. Storr-Paulsen A, Nørregaard JC, Farik G, Tårnhøj J. The influence of viscoelastic substances on the corneal endothelial cell population during cataract surgery: a prospective study of cohesive and dispersive viscoelastics. Acta Ophthalmol Scand. 2007;85(2):183-7. 21. Igarashi T, Ohsawa I, Kobayashi M, et al. Hydrogen prevents corneal endothelial damage in phacoemulsification cataract surgery. Sci Rep. 2016;6:31190. 22. Fang CEH, Mathew RG, Khaw PT, Henein C. Corneal endothelial cell density loss after glaucoma surgery or in combination with cataract surgery: a systematic review and meta-analysis. Ophthalmology. 2022;129(8):841-55. 23. Wang PX, Koh VTC, Loon SC. Laser iridotomy and the corneal endothelium: a systemic review. Acta Ophthalmol. 2014;92(7):604-16. 24. Chen HC, Lee CY, Liu CF, et al. Corneal endothelial changes following early capsulotomy using neodymium:yttrium-aluminum-garnet laser. Diagnostics (Basel). 2022;12(1):150. 25. Murata H, Kato S, Fukushima H, et al. Corneal endothelial cell density reduction: a complication of retinal photocoagulation with an indirect ophthalmoscopy contact lens. Acta Ophthalmol Scand. 2007;85(4):407-8. 26. Leem HS, Lee KJ, Shin KC. Central corneal thickness and corneal endothelial cell changes caused by contact lens use in diabetic patients. Yonsei Med J. 2011;52(2):322-5. 27. Setälä K, Vasara K, Vesti E, Ruusuvaara P. Effects of long-term contact lens wear on the corneal endothelium. Acta Ophthalmol Scand. 1998;76(3):299-303. 28. Bonanno JA. Effects of contact lens-induced hypoxia on the physiology of the corneal endothelium. Optom Vis Sci. 2001;78(11):783-90. 29. Janson BJ, Alward WL, Kwon YH, et al. Glaucoma-associated corneal endothelial cell damage: a review. Surv Ophthalmol. 2018;63(4):500-6. 30. Kristianslund O, Pathak M, Østern AE, Drolsum L. Corneal endothelial cell loss following cataract surgery in patients with pseudoexfoliation syndrome: a 2-year prospective comparative study. Acta Ophthalmol. 2020;98(4):337-42. 31. Riley MV, Winkler BS, Czajkowski CA, Peters MI. The roles of bicarbonate and CO2 in transendothelial fluid movement and control of corneal thickness. Invest Ophthalmol Vis Sci. 1995;36(1):103-12. 32. Kaminski S, Hommer A, Koyuncu D, et al. Influence of dorzolamide on corneal thickness, endothelial cell count and corneal sensibility. Acta Ophthalmol Scand. 1998;76(1):78-9. 33. Nakano T, Inoue R, Kimura T, et al. Effects of brinzolamide, a topical carbonic anhydrase inhibitor, on corneal endothelial cells. Adv Ther. 2016;33(8):1452-9. 34. Wirtitsch MG, Findl O, Kiss B, et al. Short-term effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch Ophthalmol. 2003;121(5):621-5. 35. Kwon J, Heo JH, Kim HM, Song JS. Comparison of cytotoxic effects on rabbit corneal endothelium between preservative-free and preservative-containing dorzolamide/timolol. Korean J Ophthalmol. 2015;29(5):344-50. 36. Chen W, Li Z, Hu J, et al. Corneal alternations induced by topical application of benzalkonium chloride in rabbit. PLoS One. 2011;6(10):e26103. 37. Wu KY, Hong SJ, Wang HZ. Effects of antiglaucoma drugs on calcium mobility in cultured corneal endothelial cells. Kaohsiung J Med Sci. 2006;22(2):60-7. 38. Goldstein AS, Janson BJ, Skeie JM, Ling JJ, Greiner MA. The effects of diabetes mellitus on the corneal endothelium: a review. Surv Ophthalmol. 2020;65(4):438-50. 39. Roszkowska AM, Tringali CG, Colosi P, Squeri CA, Ferreri G. Corneal endothelium evaluation in type I and type II diabetes mellitus. Ophthalmologica. 1999;213(4):258-61. 40. Kim YJ, Kim TG. The effects of type 2 diabetes mellitus on the corneal endothelium and central corneal thickness. Sci Rep. 2021;11(1):8324. 41. Zhang K, Zhao L, Zhu C, et al. The effect of diabetes on corneal endothelium: a meta-analysis. BMC Ophthalmol. 2021;21(1):78. 42. Kanawa S, Jain K, Sagar V, Yadav DK. Evaluation of changes in corneal endothelium in chronic kidney disease. Indian J Ophthalmol. 2021;69(5):1080-3. 43. Alfawaz A. Cytomegalovirus-related corneal endotheliitis: a review article. Saudi J Ophthalmol. 2013;27(1):47-9. 44. Zheng X, Yamaguchi M, Goto T, Okamoto S, Ohashi Y. Experimental corneal endotheliitis in rabbit. Invest Ophthalmol Vis Sci. 2000;41(2):377-85. 45. Erdem S, Karahan M, Ava S, et al. Examination of the effects of COVID 19 on corneal endothelium. Graefes Arch Clin Exp Ophthalmol. 2021;259(8):2295-2300. |


