Scleral lens fitting can appear daunting at first; however, if a systematic approach is taken, it can become easier to fit patients more successfully. Various challenges may occur during the fitting process such as midday fogging, conjunctival prolapse, suboptimal lens clearance over the cornea and conjunctival lumps and bumps. Here, we will cover a few cases (with a video of each) and address how to overcome fitting challenges that may arise.
 |
|
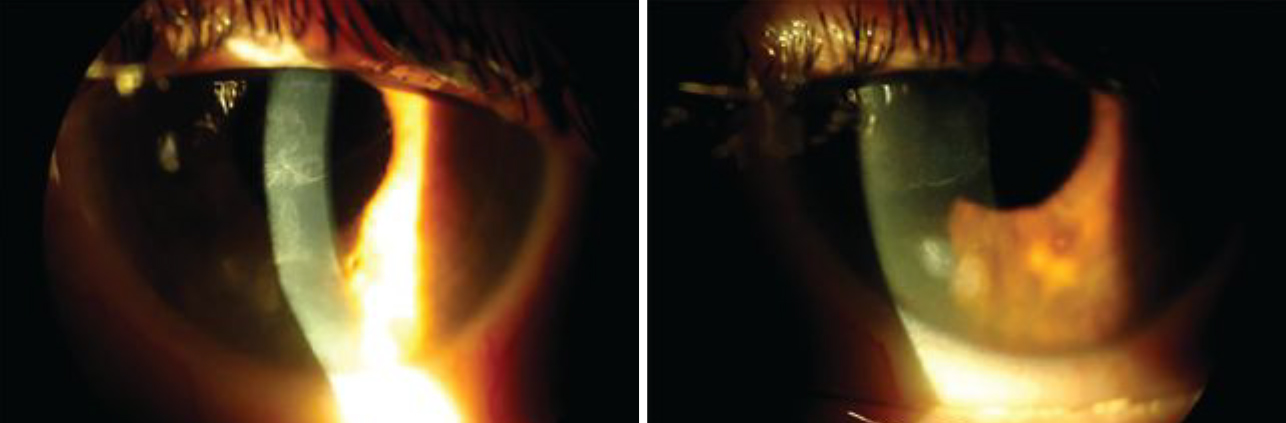
Fig. 1. Slit lamp images of the left eye show central haze on the corneal surface from recurrent epithelial defects and EBMD (left). The image on the right shows a Salzmann’s nodule located inferior nasally on the left eye. Click image to enlarge. |
Midday Fogging
This side effect occurs when there is excessive debris buildup in the scleral lens reservoir resulting in reduced vision as the day progresses. These patients typically complain of blurred vision in the middle of the day with the need to remove and reapply the lens for better vision multiple times throughout the day.
Case 1. A 56-year-old female patient was referred to the clinic for a scleral lens fitting. She had a long-standing history of epithelial basement membrane disorder (EBMD) and Salzmann’s nodular degeneration in both eyes. Her surgical history was positive for LASIK and cataract surgery. She had undergone successful cataract surgery in the right eye; however, she had complications in the left including a retinal detachment and a subsequent pars plana vitrectomy. She had a resulting surgical pupil and a neurotrophic cornea (Figure 1). With recurring epithelial defects in the left eye that were unresponsive to traditional lubrication therapy, she was referred to a corneal specialist for a scleral lens fitting for corneal rehabilitation.
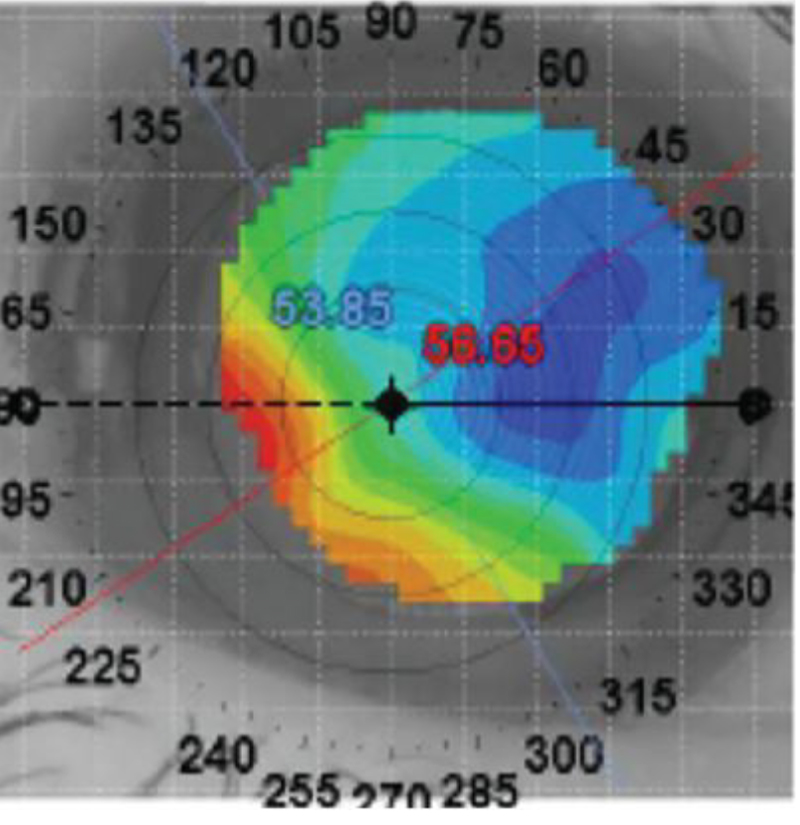
The patient had an uncorrected visual acuity of 20/25 OD and 20/30- OS. The pupil was reactive to light and had no afferent pupillary defect OD and was fixed and dilated OS. Topography revealed central corneal flattening consistent with the history of LASIK surgery in both eyes and midperipheral irregularity secondary to a Salzmann’s nodule in the left eye (Figure 2).
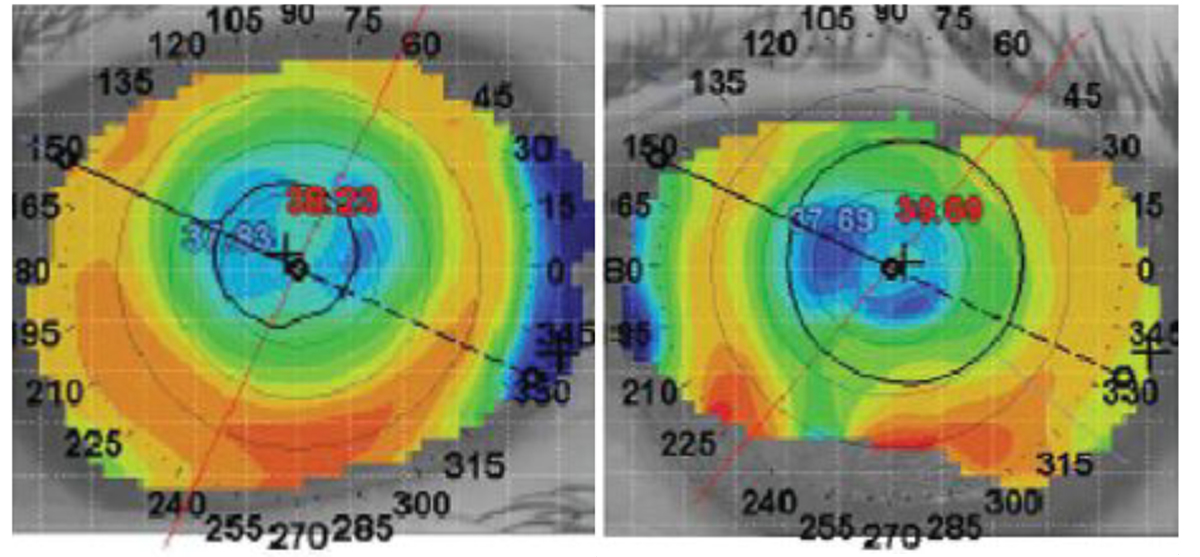 |
| Fig. 2. Topographical images of the right (left) and left eye (right). Click image to enlarge. |
An oblate scleral lens was fitted in the left eye to treat the persistent epithelial defects and preserve corneal integrity while improving visual acuity. With the initial lenses, there was significant conjunctival prolapse and midday fogging (Figure 3).
Conjunctival prolapse can act as an “oxygen sink” and reduce oxygen supply to the tissue underneath. This can propagate neovascularization in the corneal tissue. In addition, conjunctival prolapse can introduce debris from the eye surface causing midday fogging.
 |
|
Fig. 3. Excessive conjunctival tissue over the limbal region with fogging in the tear chamber. Click image to enlarge. |
Overall, there are a few ways to address midday fogging:
- Reducing limbal and midperipheral clearance can help reduce negative pressure that results in debris entrapment underneath the lens surface. This also helps with reducing prolapsed conjunctival tissue.
- Adding Celluvisc (Allergan) can increase the viscosity in the lens chamber to reduce midday fogging.
- Initiating treatments such as gel drops, ointments, lid hygiene and heat compresses can improve eye pathology. Such was the case with this patient, as those with ocular surface disease are more prone to midday fogging due to tear film imbalance.
- Managing conjunctival prolapse can help reduce midday fogging.
Lens Diameter
This is a crucial aspect of scleral lens parameter selection especially in cases of ocular surface disease. While larger-diameter scleral lenses provide lubrication over a larger area, they may not be the best option for all patients. Horizontal visible iris diameter (HVID) can be used as a tool in selecting the most appropriate lens diameter for the patient.
Case 2. A 52-year-old female patient was referred to the clinic for scleral lens management due to limbal stem cell failure. She had been using aggressive lubrication, steroids and doxycycline to manage severe surface inflammation. She presented with extreme light sensitivity and reduced palpebral fissure opening (8mm to 9mm).
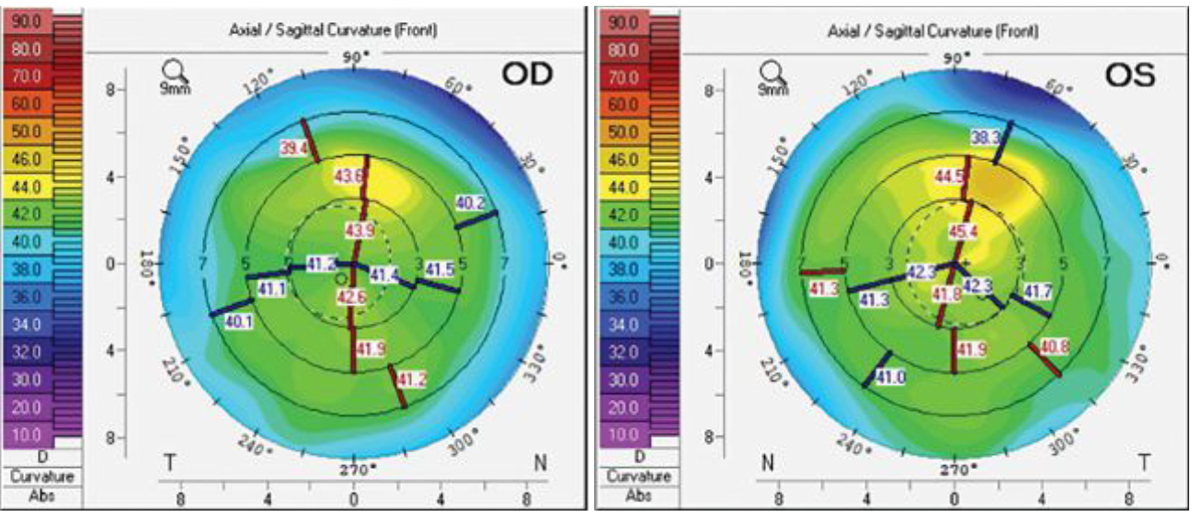
Uncorrected visual acuity was 20/30 in both the right and left eye. Extensive pannus was noted 360° with 3+ punctate epitheliopathy in both eyes. The patient had mild superior steepening from extensive pannus and scarring (Figure 4). She was advised to continue with preservative-free artificial tears, ointments, lid hygiene, warm compresses and a round of platelet-rich plasma.
 |
|
Fig. 4. Topography of the patient’s eyes shows a symmetrical profile in the right eye (left) and slight steepening superiorly in the left (right). Click image to enlarge. |
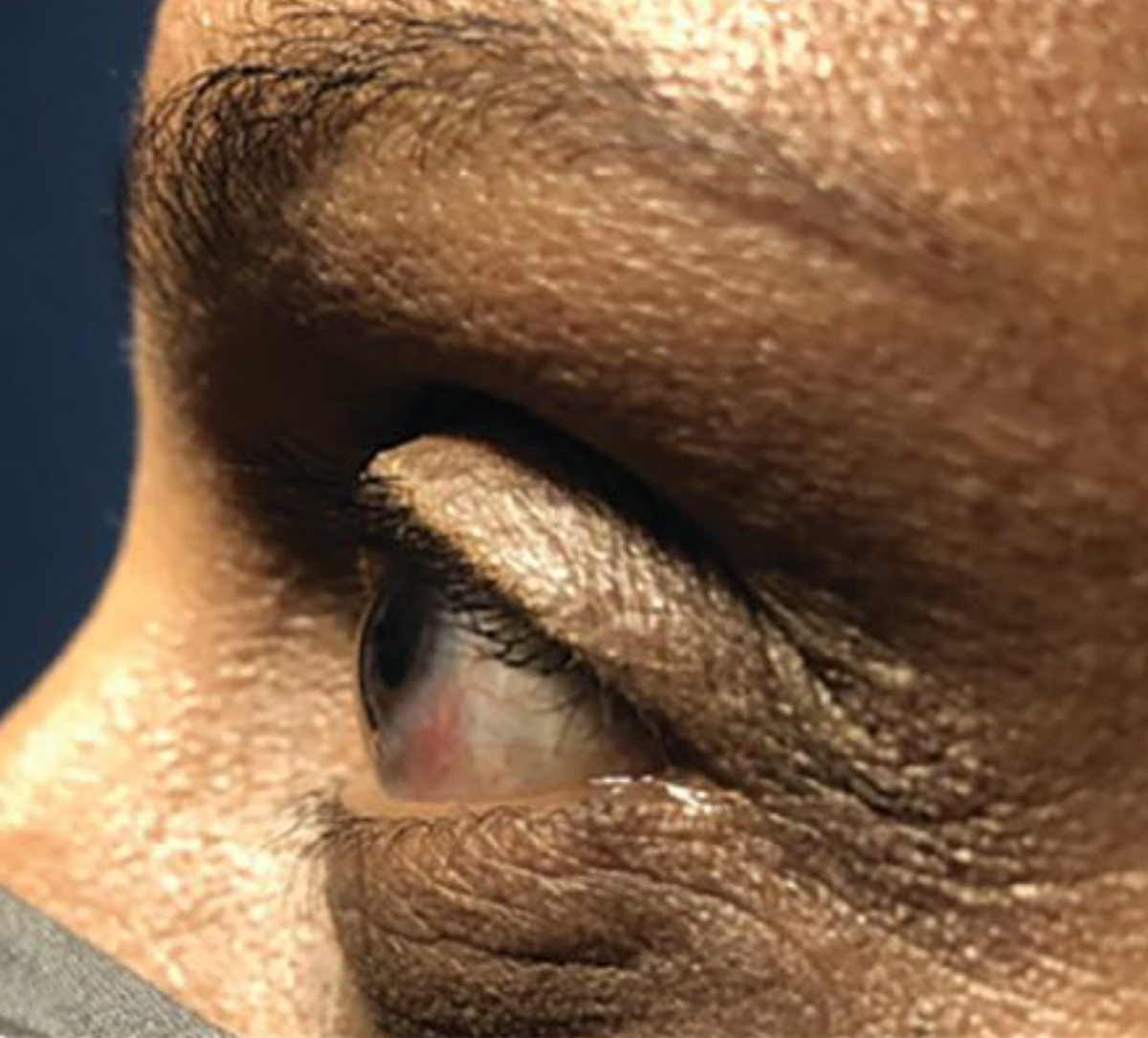
Scleral lens fitting was initiated in both eyes not for visual correction, but for ocular surface management. With a 16mm trial lens, there was extensive bubbling and poor centration. Large-diameter lenses are a great option for patients with extensive ocular surface disease as they provide constant lubrication and protection across a larger surface area. However, in cases of patients with smaller eye fissures or a smaller HVID such as in this case (10.8mm OD, 10.9mm OS), a smaller lens diameter can allow for better centration and a more optimal fit. In this case, the patient was switched to a smaller 14.8mm diameter lens with a SAG height of 3400µm due to a flatter eye profile (Figure 5).
 |
|
Fig. 5. Imaging of the patient’s left eye shows a flat corneal profile. Click image to enlarge. |
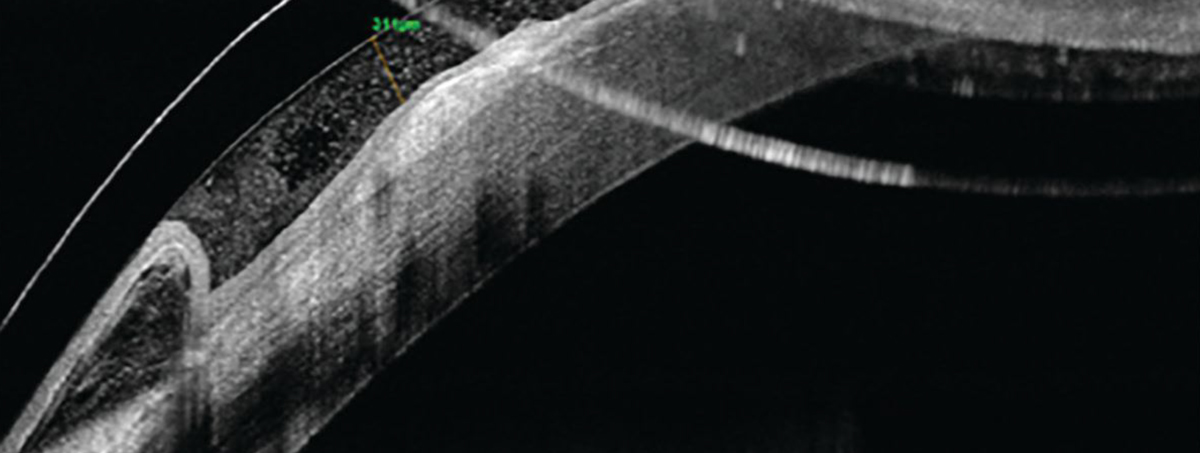
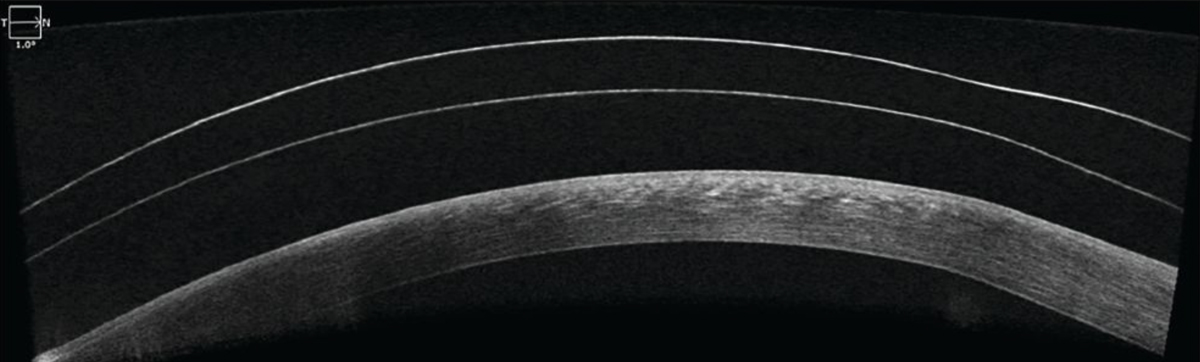
After two to three months of scleral lens wear, the patient reported better comfort, less light sensitivity and increased fissure height (Figure 6). We continued with nighttime use of gels and ointments after lens removal to prevent any discomfort and to continue hydrating the cornea for the best overall results.
Clearance
The goal of scleral lens fitting is normally to clear the cornea by 200µm to 250µm so that after settling there is sufficient clearance to prevent touch but at the same time provide sufficient oxygenation to the tissue underneath. In cases of patients with irregular corneal profiles, this may not always be possible to achieve. With advanced and asymmetric elevations, there are concerns of excessive clearance vs. minimal clearance that practitioners often run into. With advances in scleral lens technology, options such as S-map guided scleral lenses or EyePrint Pro can offer a better fit for more complicated corneal profiles.
 |
|
Fig. 6. OCT imaging of the patient’s left eye shows good central clearance over the corneal surface. Click image to enlarge. |
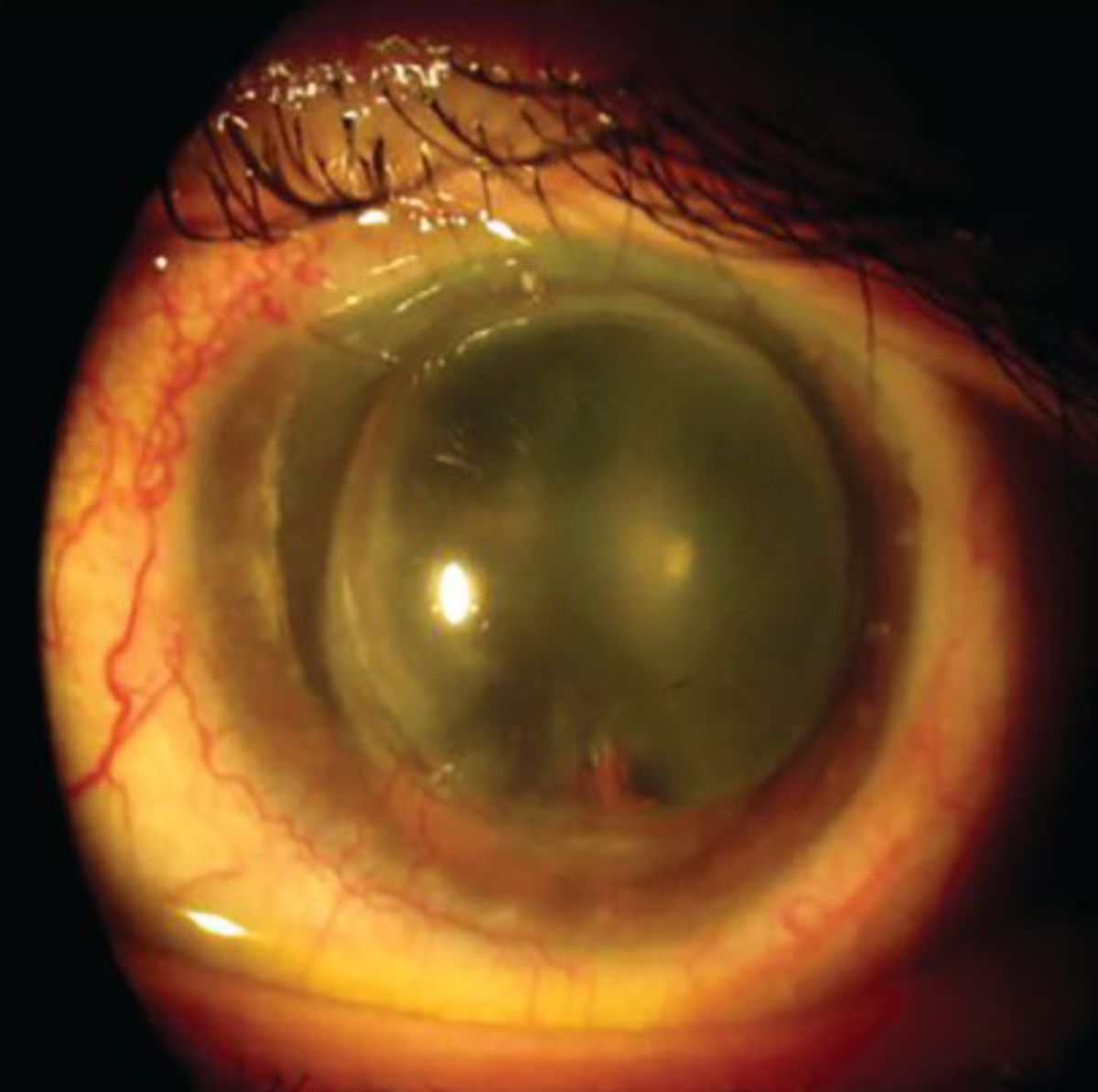
Case 3. A 61-year-old male patient presented to the clinic for a contact lens evaluation. His ocular history was pertinent for penetrating keratoplasty (PKP) in both eyes, the right graft was 30 years old and the left was 37 years old (Figure 7). The right eye had epithelial cysts at the graft-host junction, and the left presented with Urrets-Zavalia syndrome (Figure 8). In addition, the left corneal profile was prolate, as a larger-diameter corneal graft was used which resulted in severe thickening of the graft tissue at the graft-host junction in the left eye. Extensive neovascularization was also noted on the host tissue OU.
 |
|
Fig. 7. A steeper profile inferior nasally in the left eye post-PKP. Click image to enlarge. |
The patient was asymptomatic for glare, and no tinted lenses were pursued. Spectacle prescription and vision was -3.75-5.50x030, 20/20- OD and -19.00DS, 20/80 OS. The pupil was reactive to light and had no afferent pupillary defect OD and was fixed and dilated OS. The patient was fitted in scleral lenses to vault over the graft with best-corrected vision of 20/20-3 OD and 20/30 OS.
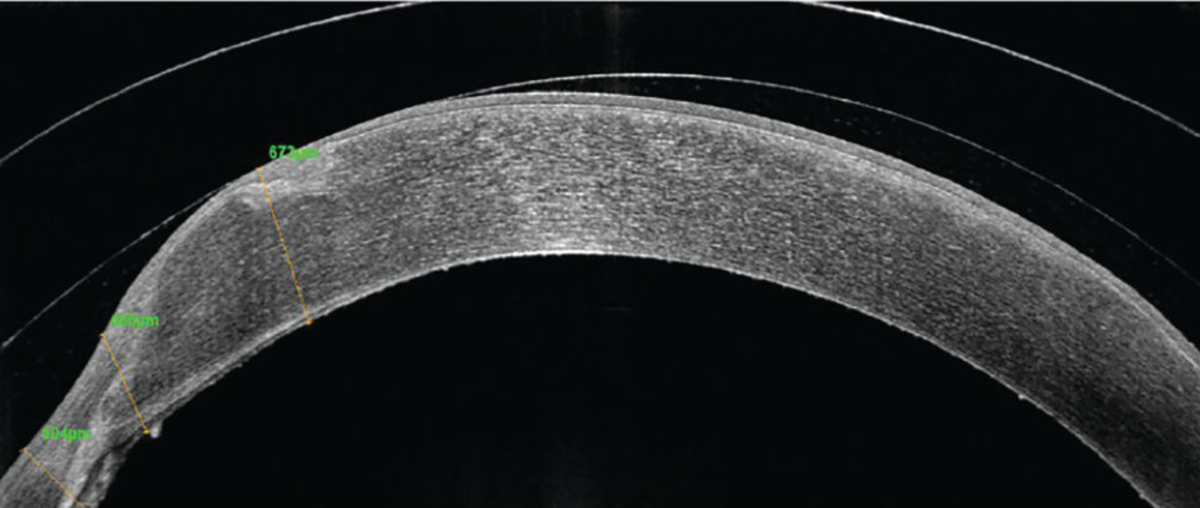
With an initial prolate lens, touch was noted nasally where the graft had the highest elevation, so vault was increased to accommodate the nasal portion and prevent any touch and mechanical trauma to the graft tissue (Figure 9). However, with clearance over the nasal portion, there was increased midperipheral and limbal clearance despite efforts to adjust the lens parameters. With excessive clearance, there is a risk of induced hypoxia to the underlying tissue and graft rejection or failure. In such cases, there are several strategies that can be employed. First, switching to a fully customized lens such as EyePrint Pro can help provide a better fit. Second, managing the peripheral curve system to obtain some tear exchange can help minimize neovascularization. In this case, since the patient declined a customized lens, a flatter peripheral curve system was used to encourage tear exchange and minimize the risk of neovascularization progression on the graft tissue.
 |
|
Fig. 8. Urrets-Zavalia syndrome in the left eye. Click image to enlarge. |
Given the high minus prescription and concerns about peripheral thickness of the lens limiting oxygen supply at the limbal area, the issue of hypoxia was combated with selection of a hyper-Dk material, thinner lens design to reduce the average thickness of the lens, reduced tear layer underneath the scleral lens, reduced overall wear time and flatter peripheral curve system.
Rigid gas permeable lenses are tremendously beneficial for patients with postoperative residual refractive error to aid vision rehabilitation. However, contact lens use increases the risk of infections, microcystic edema and neovascularization that can potentiate graft rejection. Therefore, it is important to understand the complications resulting from scleral lens wear to minimize the risk of graft failure. It is crucial to routinely monitor patients for clarity and compactness of the graft, assess the extent and caliber of neovascularization with photo documentation and evaluate the endothelial cell count and other adverse events such as the stability of epithelial cysts and thickening or thinning at the graft-host junction. Routine monitoring is important in ensuring graft health and detecting the earliest signs of graft failure.
 |
|
Fig. 9. OCT imaging of the left eye with scleral lens wear shows nasal touch on the graft tissue. Click image to enlarge. |
Lumps and Bumps
Scleral lens landing zone curvature is crucial in optimizing the fit and enhancing patient comfort. Conjunctival growths such as pinguecula and pterygium and surgical blebs in glaucoma patients can pose a challenge in scleral lens fitting. Employing notches and other peripheral modifications can overcome these concerns and provide an overall improved scleral lens fit.
Case 4. A 34-year-old Hispanic male presented to the clinic for a specialty contact lens examination and evaluation. The patient had previously been prescribed corneal gas permeable lenses. However, he reported that, due to contact lens discomfort, he only used contact lenses occasionally. The patient’s presenting unaided visual acuity at distance was 20/50+2 with a pinhole acuity of 20/20-2 OD and 20/250 with a pinhole acuity of 20/70-1 OS.
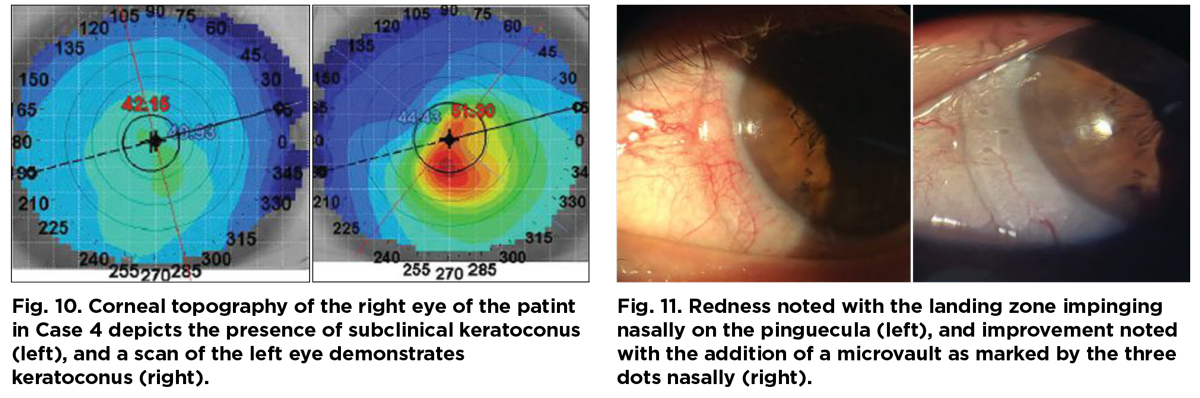
His spectacle prescription at the time of the visit was -1.25-1.25x042 with a visual acuity of 20/20 OD and -1.25-7.50x122 with a visual acuity of 20/50- OS. Corneal topography demonstrated asymmetric inferior corneal steepening consistent with keratoconus (Figure 10).
 |
| Click image to enlarge. |
The ectasia was not advanced, and only a slight protrusion was present in the left eye. As such, a prolate lens design was picked for the trial lens. The patient had nasal pinguecula which resulted in impingement and rebound redness upon removal of the trial lens after 40 minutes of in-clinic lens wear (Figure 11). In this case, a microvault was ordered over the pinguecula for better alignment and comfort for the patient.
For other ways to alleviate such concerns:
- Increase the lens diameter to go over the pinguecula.
- Decrease the diameter to avoid contact with the pinguecula.
- Add a microvault.
- Add a notch to avoid interaction with the pinguecula.
Scleral lenses play a significant role in the management of complex ocular surface pathologies. Notches and a microvault built into the lens can allow for better alignment with conjunctival toricity and obstacles.
Takeaways
Sclerals are an excellent option for visual and ocular surface rehabilitation for patients with ocular pathologies. By following a systematic approach to lens fitting and parameter manipulation, you can troubleshoot a wide variety of issues that may arise. When initially starting to fit lenses, it is important to lean on fitting guides and consultants. With increasing complexity of cases, such as when working through advanced anatomical challenges where traditional scleral lenses may not be a sufficient option, it is important to expand your options to profilometry and impression technology to optimize the lens fitting.
Dr. Bedi’s optometry practice in Mississauga, ON, focuses on specialty contact lens fitting for corneal pathology, dry eye management and myopia control. She is a Fellow of the Scleral Lens Society and the American Academy of Optometry. She has no relevant financial interests to disclose.


