We see dry eye disease in our practices on a daily basis. And, because aging is a major risk factor for dry eye, we will see more ocular surface disease in our practices, as Baby Boomers continue to age.
Considerations in Tear Use
Practitioners have a considerable armamentarium of therapeutic approaches that can be successfully utilized to address dry eye, enabling us to eliminate exacerbating factors where possible. As an early treatment step, regular use of artificial tears can provide relief to dry eye patients. It is essential, however, to specify the recommended brand and frequency of use so therapy success can be accurately measured.
Another crucial reason to be very specific with patients about artificial tears: Formulations that contain benzalkonium chloride (BAK) can actually aggravate dry eye and further exacerbate signs and symptoms of the disease. This can be problematic in patients who are also using topical medications for chronic conditions, such as glaucoma.
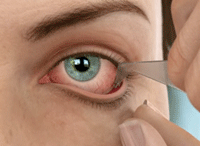
The Lacrisert insert must be placed properly in the inferior cul-de-sac. Courtesy of Aton Pharma.
Pros and Cons of Preservation
Preservatives are utilized in many multi-dose ophthalmic preparations to maintain sterility. But, clinical studies have shown that preservative agents can cause dose-dependent toxic effects that compromise tear film stability and cause damage to the cornea and conjunctiva.1-3
These ocular side effects can translate into symptoms such as stinging, burning and dryness. Preservatives are also associated with allergic reactions that can occur when the eye is hypersensitized by repeated, long-term use of certain preserved eyedrops.4,5
Considerable evidence also shows benzalkonium chloride’s disruptive effect on the tear film.6,7 The preservative has a detergent effect on the lipid layer of the tear film. This reduces the lipid layer’s stability and causes excessive evaporation, which results in increased ocular dryness.8
The impaired protective layer also predisposes the eye to inflammation and conjunctival metaplasia. In addition, preservatives have destructive effects on the mucous gland. This reduces the quantity of goblet cells and lowers production of the protective mucin layer.9 Additional effects of preservative toxicity include conjunctival epithelium inflammation and subconjunctival fibrosis.4
Go Dropless
The utilization of multi-dose artificial tears when beginning dry eye therapy is a well accepted practice among eye care practitioners. But, if artificial tear use increases to more than four or six drops per eye per day, practitioners will usually resort to artificial tear preparations in preservative-free unit-dose vials to minimize excessive exposure, even to the mildest preservatives.
One alternative to artificial tears is a hydroxypropyl cellulose ophthalmic insert, such as Lacrisert (Aton Pharma). This can be utilized for dry eye patients who use tears at least several times per day. Lacrisert is a sterile, preservative-free, sustained-release prescription insert for use in patients with moderate to severe dry eye. It is self-administered by the patient into the inferior cul-de-sac, where it slowly dissolves over a 24-hour time period. During this time, it releases demulcents into the tear film. This slow release of demulcent allows the insert to retain moisture, stabilize the tear film and lubricate the eye.
In a 520-patient study, treatment with Lacrisert over four weeks resulted in significant reductions in mean severity of dry eye symptoms and significant improvements in mean ocular surface disease index (OSDI) scores by more than 21%—better than results seen with other dry eye therapies.10 Treatment with Lacrisert also resulted in significant improvement in quality of life measures. The most commonly reported adverse event was blurred vision in 8.7% of patients.10
Previous clinical studies have demonstrated the insert’s ability to improve dry eye symptoms by increasing tear film break-up time and decreasing rose bengal staining.11-16
This technology may benefit those patients who have used artificial tears more than four times per day and are inconvenienced by the frequent instillation required. Most patients find the insert comfortable and unnoticeable, but it is essential that you train them regarding proper use. If the insert is not placed properly in the inferior cul-de-sac, it can fall out, or the patient may feel a foreign body sensation.
When I prescribe Lacrisert to a patient, I will place it in the patient’s eye myself and have a staff member call them to follow up the next day. If the response is positive, the patient is scheduled to return for training on how to self-insert the Lacrisert with the included flexible plastic applicator.
Initial training time on the use of the insert is usually relatively short and significantly shorter than an initial contact lens instruction. Once proficient, patients can insert Lacrisert very quickly. Many find it more convenient than using artificial tears several times a day, especially those patients with busy lifestyles.
Most patients, especially those who have been trained properly, have reduced their use of artificial tears and respond well to the treatment. A chart review study of Lacrisert patients shows the median length of therapy to be more than five years, with nearly 65% using the inserts for at least two years.17 Long-term use is practical because of the insert’s once-daily instillation and the ability to use it concurrently with other dry eye treatments. Contraindications can occur if the patient is hypersensitive to any component of the product. The 8.7% who experienced the blurred vision in the study discontinued use as a result.
I recommend patients use this product in the evening if they experience discomfort from daily insertion. For many, this will provide some relief. Lacrisert poses no contraindications with other medications.
Uncomfortable Lens Wear
We have many effective options for patients with contact lens discomfort at our disposal. Significant advances in contact lens materials have allowed many previously intolerant contact lens wearers to return to lens wear. Most notably, the introduction of silicone hydrogel contact lenses and advances in polymer technology have provided comfortable wearing experiences for formerly intolerant lens wearers. These modern materials provide many benefits, but their most significant effect is increased oxygen delivery to the cornea, which has resulted in regression of neovascularization, decreased corneal edema and decreased limbal hyperemia when secondary to corneal hypoxic stress. But, with their widespread adoption and use, practitioners must understand that these materials interact with contact lens solutions very differently than many of their hydrogel predecessors.
Likewise, contact lens care solutions are complex; they perform a multitude of tasks that ultimately contribute to either success or failure of contact lens wear. A delicate balance exists between disinfection efficacy and the ability to be gentle to the ocular surface. Research has demonstrated differences in biochemical dynamics between various contact lens care systems.18-19 For example, it has been demonstrated that Polyquad/Aldox-preserved solutions retain much of their disinfecting capabilities under a variety of extreme testing conditions.18-19
Research has also demonstrated interactions between various contact lenses and solutions that seem to produce transient corneal staining responses. This response seems to occur least frequently with hydrogen peroxide systems and Polyquad/Aldox-preserved solutions.20-21
There are a multitude of factors to consider when fitting contact lenses, including recommending the appropriate lens care solution. Practitioners should use their experience and clinical literature to recommend the most appropriate combinations of materials and solutions.
When switching preservative systems due to sensitivity, patients should change their contact lens case as well; this prevents any transfer of preservatives from the previous care system. If sensitivity still exists after the switch, a hydrogen peroxide-based system may be utilized. Note that with peroxide systems, patients should be well informed of the ill effects of noncompliance, as this may lead to significant corneal irritation and pain if used incorrectly or if the solution is not completely neutralized.
Optimizing the lens material and care system will often successfully reduce or eliminate discomfort issues. If the patient needs re-wetting agents, keep in mind the clinical considerations that were discussed previously with the use of artificial tears. Treating underlying dry eye disease in those patients who experience significant discomfort with contact lens wear will also be critically important in maintaining successful contact lens wearers.
Hydroxypropyl cellulose inserts, such as Lacrisert, may be a valuable tool in this patient population as well. A subset of 86 contact lens wearers in a large-scale study experienced measurable improvements in many areas, including a 23.2% improvement in OSDI scores.22 This technology offers patients an additional means to improve comfort—especially for those who have exhausted all other options. In my experience, Lacrisert has worked well for patients who benefit visually but have comfort issues with specialty lenses (usually gas-permeable lenses or hybrid designs).
Final Thoughts
Dry eye disease is a complex disease process that affects many of our patients. The varying severity of presentations and the number of clinical factors that need to be considered in the cause and management of this disease make it a challenging landscape for both our contact lens wearers and non-contact wearers.
It is incumbent upon the eye care practitioner to understand the magnitude of the factors that can affect this condition, and to modify what we can to enhance the health of the ocular surface and decrease patient symptoms. Preservatives, although beneficial in providing a sterile option in a multi-dose container for many of our patients, can also have certain unwanted effects on the ocular surface.
Practitioners must strategically select those treatment options that contain gentle preservatives and minimize their use when necessary. Optometrists have the ability to alter the effects these treatment options may have and, in turn, optimize our patients’ ocular health and comfort.
Dr. Brujic is a partner of Premier Vision Group, a multi-location group
practice in Northwest Ohio. He lectures nationally on contemporary
topics in eye care. Dr. Brujic has received honoraria from Aton Pharma,
Inc., for consulting services.
1. Noecker R. Ophthalmic preservatives: Considerations for long-term use in glaucoma patients with dry eye or glaucoma. Review of Ophthalmology. 2001 June.
2. Cha SH, et al. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro Clin Experimental Ophthalmology 2004;32:180-184.
3. Broadway DC, I. Grierson I, O’Brien C; Hitchings RA. Adverse effects of topical antiglaucoma medication. Arch Ophthalmol. 1994;112:1437-1445.
4. Baudouin C. The Ocular Surface in Glaucoma, Cornea, Volume 28, Number 9, Suppl. 1, October 2009.
5. Baudouin C. Allergic reaction to topical eye drops. Curr Opin Allergy Clin Immunol. 2005;5:459–63.
6. Kuppens EV, de Jong CA, Stolwijk TR, et al. Effect of timolol with and without preservative on the basal tear turnover in glaucoma. Br J Ophthalmol. 1995;79:339–42.
7. Nuzzi R, Finazzo C, Cerruti A. Adverse effects of topical antiglaucomatous medications on the conjunctiva and lachrymal response. Int Ophthalmol. 1998;22:31–35.
8. Detry-Morel M. Side effects of glaucoma medications. Bull Soc Belge Ophtalmol. 2006;299:27–40.
9. Herreras JM, Pastor JC, Calonge M, et al. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992;99:1082–88.
10. Koffler B, LACRISERT® (hydroxypropyl cellulose ophthalmic insert) Significantly improves symptoms of dry eye syndrome and patient quality of life. 2009 Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO), poster 4660/D904.
11. Høvding G, Aasved H. Slow-release artificial tears (SRAT) in dry eye disease: Report of a preliminary clinical trial. Acta Ophthalmol. 1981;59:842-46.
12. Breslin C, Katz J, Kaufman H, Katz I. Slow-release artificial tears. Symposium on Ocular Therapy. 1977;10:77-83.
13. Katz J, Kaufman H, Breslin C, Katz I. Slow-release artificial tears and the treatment of keratitis sicca. Ophthalmol. 1978;85:787-93.
14. Lamberts D, Langston D, Chu W. A clinical study of slow-releasing artificial tears. Ophthalmol.1978;85:794-800.
15. Werblin T, Rheinstrom S, Kaufman H. The use of slow-release artificial tears in the long-term management of keratitis sicca. Ophthalmol. 1981;88(1):78-81.
16. Hill J. Slow-release artificial tear inserts in the treatment of dry eyes in patients with rheumatoid arthritis. Br J of Ophthalmol. 1989;73:151-54.
17. Wander A, Koffler, B. Retrospective case series study of the c ophthalmic insert. Ocular Surface. 2009;vol ,no.3:154-61.
18. Rosenthal RA, Dassanayake NL, Schlitzer RL, Schlech BA, Meadows DL, Stone RP. Biocide uptake in contact lenses and loss of fungicidal activity during storage of contact lenses. Eye Contact Lens. 2006 Dec;32(6):262-6.
19. Rosenthal A, Henry C, Buck S, Bell W, Stone R, Schlech, B. Extreme testing of contact lens disinfecting products: multi- purpose solutions face pathogens and environ-mental extremes to replicate real world contact lens experience. Contact Lens Spectrum, July 2002.
20. Andrasko G, Ryen K. Corneal staining and comfort observed with traditional and silicone hydrogel lenses and multipurpose solution combinations. Optometry. 2008 Aug;79(8):444-54.
21. Jones L, MacDougall N, Sorbara LG. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optom Vis Sci. 2002 Dec;79(12):753-61.
21. Curwen, J et al. Effect of Lacrisert on the signs and symptoms of dry eye in contact lens wearers, ARVO 2009.


