 |
The Consequences of Corneal Endothelial Compromise
Optometrists must recognize the signs to ensure proper diagnosis and treatment.
By Bhawan Minhas, OD
Release Date: February 15, 2021
Expiration Date: February 15, 2024
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group.
Educational Objectives: After completing this activity, the participant should be better able to:
- Describe the pathophysiology of endothelial diseases.
- Identify and monitor the common warning signs of endothelial compromise.
- Diagnose endothelial diseases.
- Evaluate patients when a specular microscope is unavailable.
Target Audience: This activity is intended for optometrists engaged in primary care of the anterior segment of the eye.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Bhawan Minhas, OD, FAAO, Salus University/Pennsylvania College of Optometry.
Credit Statement: This course is COPE approved for 2 hours of CE credit. Course ID is 70781-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Authors: Dr. Minhas has no financial disclosures.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
The simple yet sophisticated organization of the corneal layers—the epithelium, Bowman’s membrane, stroma, Descemet’s membrane and endothelium—results in transparency, allowing visual rays to reach the retina. The corneal endothelium, the most posterior layer, is also the most fragile. This hexagonal, non-replicating monolayer of tissue is able to maintain balanced corneal hydration through the help of ion channels that enable fluid transport.1
This intricate balance can be disrupted by a variety of factors including age, injury or trauma, genetic dystrophies and secondary degenerations. A precise understanding of normal anatomy and physiology, as well as the application of various examination techniques, can aid the clinician in the evaluation of this thin but mighty corneal layer.
| The Cornea Close Up The complex balance of the five recognized layers of the cornea is fundamental to maintaining visual integrity. Although Dua’s layer has been detected and reported as a discrete pre-Descemet’s entity, its relevance has been limited to visualization during corneal graft procedures, namely deep anterior lamellar keratoplasties.2,3 Generally, the corneal epithelium functions as a barrier to invaders from the external world, the stroma maintains adequate corneal thickness and shape in order to provide refractive power and the endothelium maintains hydration of the stroma and nutrition of cells. With the exception of oxygen, corneal nutrients are extracted primarily from the aqueous humor. This necessitates the use of both passive diffusion and active transport of molecules through various enzymatic transport systems on the apical and basolateral flanks of the endothelial layer.4 |
The Pump-Leak Process
The dimensions of an average corneal endothelial cell are 18μm to 20μm wide, 4μm to 6μm thick and 7μm in diameter with a six-sided shape comprising the majority of cells.4,5 These cells are not known to undergo mitosis in-vivo; on the contrary, they gradually undergo apoptosis during their lifetime, with a critical mass of approximately 400 to 700 cells per mm2 that is necessary to maintain tissue transparency in normal individuals with average intraocular pressure (IOP).6,7 Due to this natural, progressive decline, the surface of the remaining cells may morph to compensate the subsequent changes to the monolayer. It is well documented that the average adult endothelium maintains a cell density of approximately 2,500 to 3,000 cells per mm2.8
The endothelium contributes to visual clarity and corneal deturgescence using a specialized pump-leak system. The “pump” aspect pertains to active transport properties of membrane-bound channels and the intercellular junctional complexes. These compensate for stromal swelling that occurs as a result of the “leak” created to maintain corneal hydration and nutrition via hydrostatic pressure of the aqueous and the oncotic pressure of the cornea.6
Active transport mechanisms of the endothelium are primarily achieved by the Na+, K+, HCO3- and Cl- ions along with carbonic anhydrase.9 Active exchange of these substances gives rise to an ionic gradient between the stroma and aqueous humor, which allows for the extraction of water from the cornea. The pumping process is further facilitated by lateral interdigitations, gap junctions and tight junctions on the lateral borders of adjacent cells.9
As discussed above, though normal throughout the course of life, endothelial cell death potentiates a problem in maintaining endothelial cell clarity where the pump function is compromised. Centripetal migration and stretching to compensate for lost cells in the absence of regeneration naturally causes changes in cell size and shape that then correlate with pump dysfunction. Specifically, polymegethism (increase in cell size) and pleomorphism (change in cell shape) are both related to a reduction in the ability of the endothelium to dehydrate the cornea.10
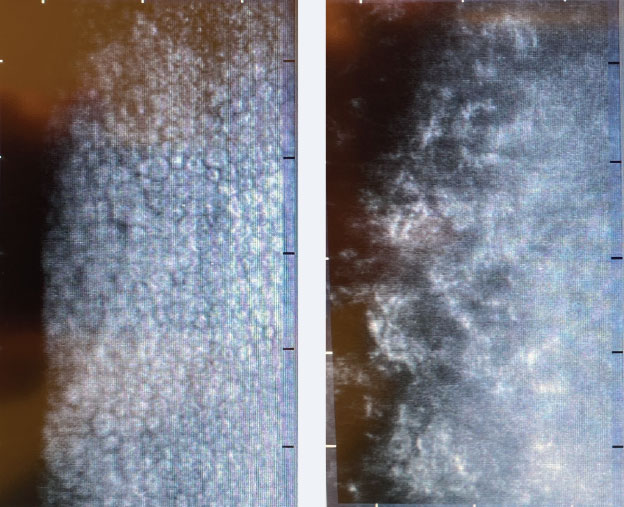 |
| Fig. 1. Non-contact specular microscopy of a normal corneal endothelium (left) and of a patient with Fuchs’ dystrophy (right). Click image to enlarge. |
Endothelial Evaluation
Comprehensive evaluation of the corneal endothelium is crucial to ensure proper diagnosis and care. The various ways to conduct this evaluation are discussed below.
Specular and confocal microscopy. While most clinicians do not have access to these tools, these visualization instruments are important, revealing that the endothelium is quite complex and maintains a three-dimensional shape that counterparts its multifarious role. Specular and confocal microscopy allows for quantitative evaluation of the endothelium for diagnostic purposes, monitoring for progression and assessing response to treatment in endothelial cell pathology (Figure 1).
The honeycomb-like mosaic of the endothelium is best evaluated through a specular microscope. This hexagonal shape, however, is only visible on the apical surface of the cell, which is in contact with the anterior chamber.5
As it pertains to the remaining surfaces, three-dimensional confocal microscopy has enabled researchers to categorize and map the lateral and basilar aspects of endothelial cells. Lateral membrane expansions with multiple membrane folds on the lateral aspects indicate a complex network of cellular interdigitations.5 Additionally, these membrane expansions have been shown to increase in number and length as they move from the apex to the basilar surface.5
Interestingly, a smartphone-based microscopy system using an iPhone has shown initial promise in visualizing the endothelium in a sub-cellular resolution as a possible alternative in rural settings.11 Though many preliminary studies have revealed the qualitative and quantitative abilities of smartphone specular microscopy in analyzing the most posterior corneal layer, further refinement to standardize light sources and automate analysis is needed before this can become commercially available.12
More widely used and readily available methods to examine the corneal endothelium in the routine clinical setting can be distinguished into three strategies: slit lamp biomicroscopy techniques, indirect measures of adjacent stromal thickness and anterior segment optical coherence tomography (AS-OCT).
Slit lamp biomicroscopy. This is often the workhorse of visualizing corneal health and monitoring for pathology of the endothelium. Many times, it is one of the only visualization tools a clinician may have access to. This tool can be infinitely useful for perceptive clinicians if used appropriately. Initial analysis of observed corneal pathology is performed through the use of an optic section. This cross-sectional observation technique aids in determining anterior to posterior depth and specific corneal location of an abnormality. Additional lighting techniques, such as specular reflection, direct and indirect lighting and retroillumination may then be employed.
As a primary means of visualizing the endothelium in a focal location, specular reflection of the endothelium at a high magnification allows direct visualize of a discrete number of cells. Best practices include keeping the area of illumination small, reducing extraneous reflected light, utilizing the highest possible magnification and attempting to view monocularly. In this method, individual cells appear as a hexagonal mosaic and guttae; droplet-like accumulations of collagen will appear as dark, non-reflective areas. Additionally, both direct and indirect illuminations are helpful, especially under high-powered magnification, in viewing pigmented abnormalities of the endothelium (Figure 2). Sclerotic scatter is best used to visualize even subtle stromal edema that may result from endothelial dysfunction.1 Finally, retroillumination in the setting of a dilated pupil can help determine the extent and severity of endothelial pathology (Figure 3).
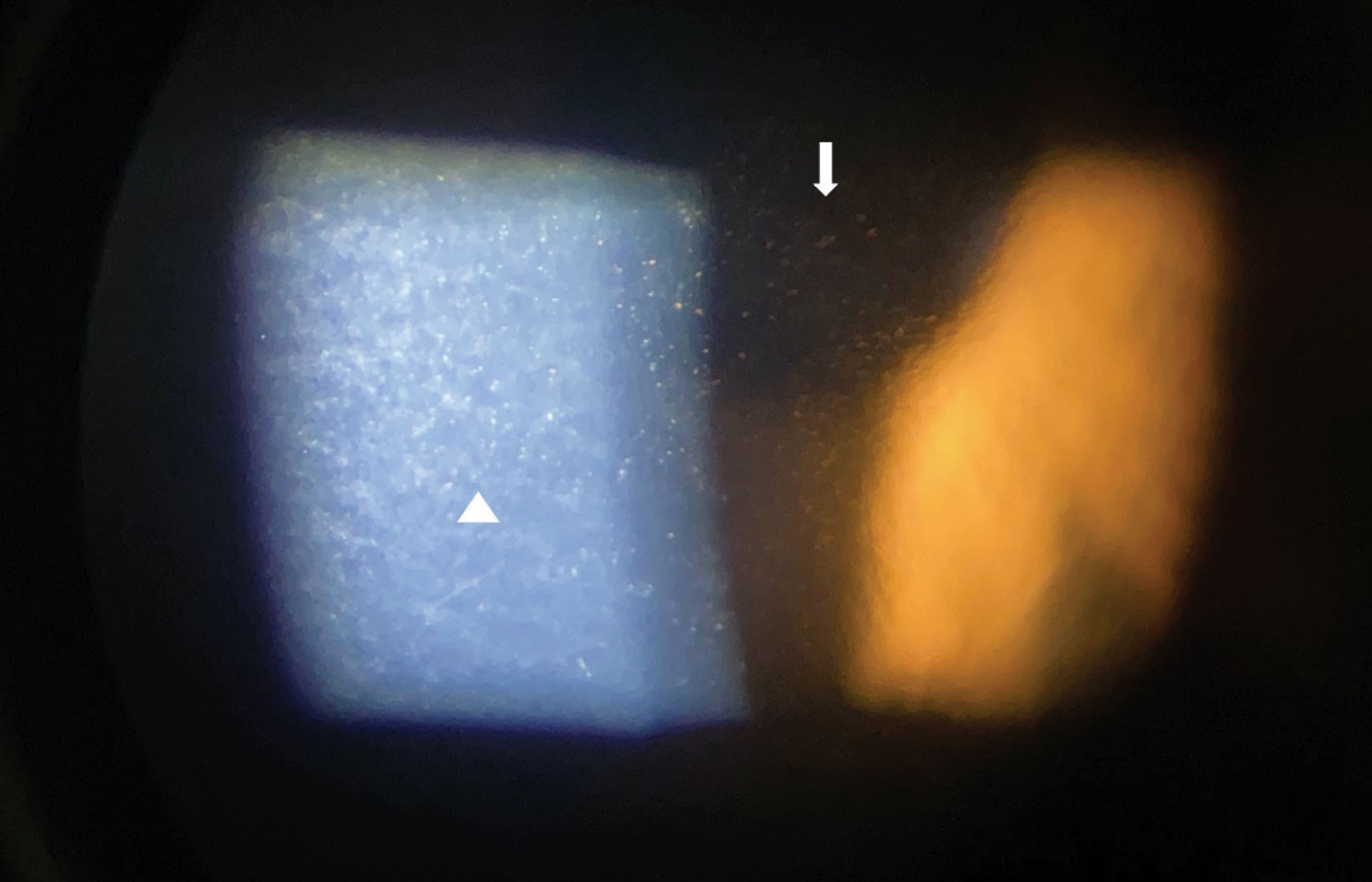 |
| Fig. 2. Slit lamp imaging through an iPhone X of pigmented corneal guttata in a patient with Fuchs’ endothelial dystrophy with lesions viewed in high magnification in direct (triangle) and indirect (arrow) illumination. Click image to enlarge. |
Indirect measurement of stromal thickness. Due to the endothelium’s role in maintaining stromal clarity, an indirect measure of endothelial pump integrity can be ascertained through the measurement of corneal thickness. As the pump-leak process breaks down, such as in Fuchs’ endothelial dystrophy, the cornea has the propensity to thicken as a result of edema within the stromal layer. Even in cases where the edema is not readily observed through clinical exam, studies have demonstrated that there is still an increase in measurement of central corneal thickness in all grades of Fuchs’.13 Thus, it can be extrapolated that central corneal thickness can be used as an indirect, quantitative parameter to help monitor progression of endothelial loss in pathology.13 It is important to note that this relationship has been established in endothelial diseases, but not in relation to the natural, age-related degradation of endothelial cells.14,15
Pachymeters measure corneal thickness. The most common type of ultrasound pachymetry is a handheld portable device. While this device is fairly cost-effective, it is particularly dependent on the proper positioning of the probe perpendicularly to the corneal surface.
More modern devices that include pachymetry measurements are able to take accurate measurements without contact but come with a higher price tag. Optical devices include AS-OCT such as Visante (Zeiss), slit-scanning corneal topography such as Orbscan (Bausch + Lomb) and Scheimpflug systems such as the Pentacam (Oculus) and Galilei (Ziemer). Scheimpflug devices demonstrate a higher repeatability of thickness measurements in advanced corneal pathology vs. slit-scanning devices.16 Additionally, the latter devices allow clinicians to obtain pachymetry measurements of the entire corneal surface and monitor for variations as opposed to a single, central locale as seen in ultrasonic devices.
AS-OCT. This high-resolution cross-sectional modality is used for a vast array of anterior segment pathologies and can be used for postoperative monitoring and management of endothelial keratoplasties, such as Descemet’s stripping endothelial keratoplasty (DSEK) and Descemet’s membrane endothelial keratoplasty (DMEK).17 It can also be used to visualize in vivo biomarkers for various corneal diseases in a noninvasive fashion. Altered reflectivity patterns within Descemet’s can be visualized in Fuchs’ endothelial dystrophy.18 Furthermore, AS-OCT can be used to directly image endothelial disruptions, the presence of inflammatory cells or keratic precipitates, and stromal edema or opacities with more detail and accuracy.19
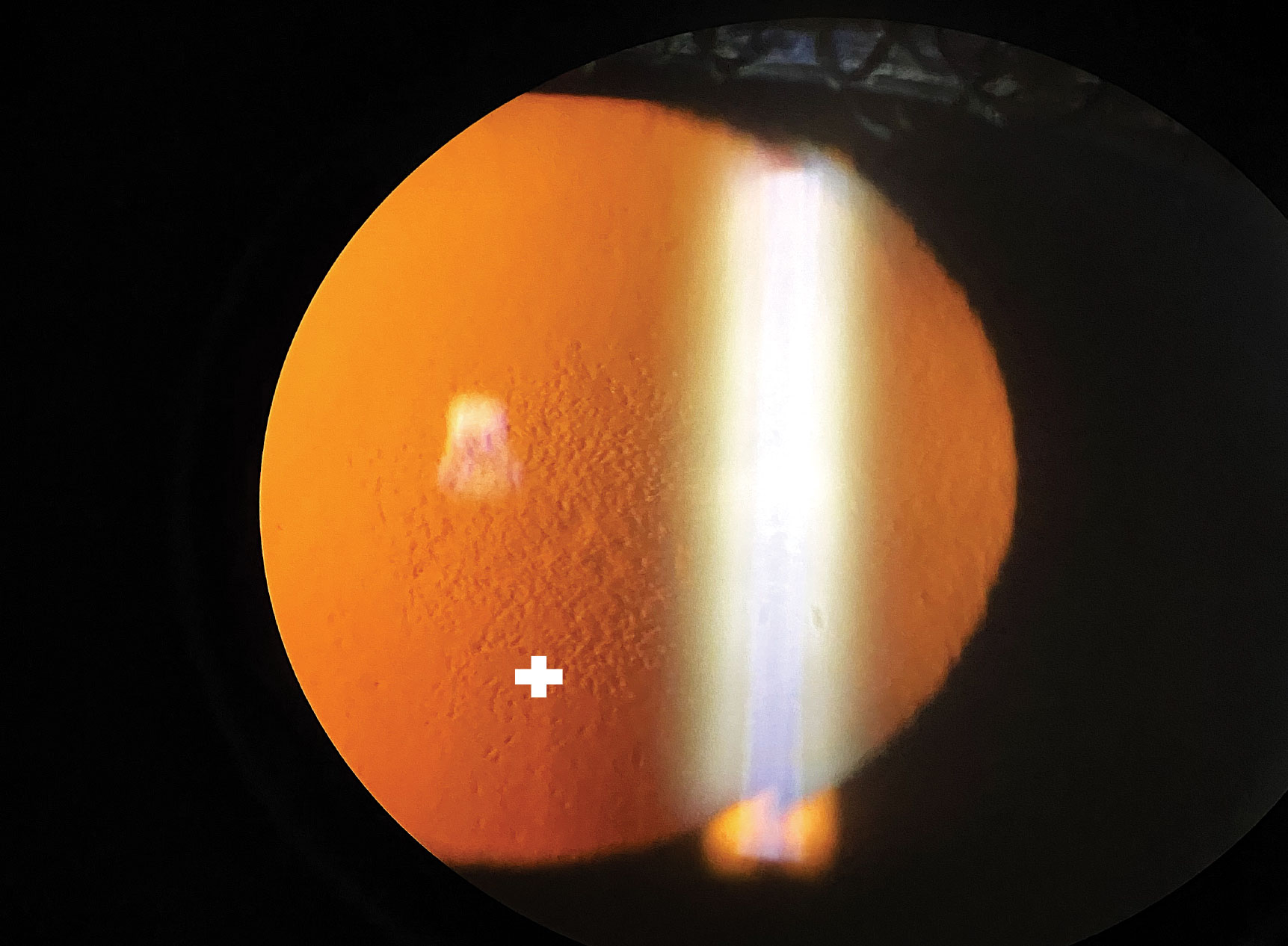 |
| Fig. 3. Slit lamp imaging using an iPhone X of central guttata in a patient with Fuchs’ endothelial dystrophy with lesions viewed in retroillumination (plus sign). Click image to enlarge. |
Warning Signs
The endothelium lies adjacent to Descemet’s layer and relies on a healthy and intact basement membrane and, subsequently, the production of the extracellular matrix.20 Although the endothelium is not in direct contact with the stroma, should it fail in maintaining optimal hydration, the uniform spacing of the stromal fibers that is typically coordinated by glycosaminoglycans collapses, causing increased scattering of incident light due to non-uniform spacing and loss of transparency.
Early warning signs of endothelial dysfunction can include pleomorphism, polymegethism and guttae, but these can also be present in normal eyes. Thus, the most diagnostic sign of corneal decompensation is typically edema and, in more advanced stages, bullae formation. An affected patient may initially report blur, discomfort and even severe pain as conditions worsen. In chronic cases, permanent scarring may occur.
Pathophysiology
To ensure timely and proper diagnosis, optometrists should be aware of the various factors that can disrupt the corneal endothelium.
Age. The natural and gradual reduction in endothelial cell density occurs at a rate of 0.56% to 0.60% per year.21,22 On the contrary, Descemet’s is known to steadily increase throughout life due to the continuous secretion of the basement membrane. It is important to distinguish the rate of change due to normal aging from that which is more prominent in pathology.
Injury/trauma. Corneal endothelial injuries from trauma are clinically observed as a “snail-track” or winding gray lines. A relatively common example of this is found in the incisional scar following extracapsular cataract extraction. Rapid focal distortion of the endothelium is often the culprit behind the linear opacities and is similar to the injury received during large-incision surgeries from excessive corneal bending.9
The endothelium responds to injury in three stages: (1) initial coverage of the wound via migration of adjacent cells, (2) re-establishment of tight junctions and return to normal corneal thickness and (3) remodeling of endothelial cells into regular hexagonal shape.9 The initial stage compensates for the markedly reduced number of tight junctions and ability to carry out optimal pumping and maintain transparency. The subsequent stages compensate by increasing the quantity of tight junctions and endothelial remodeling, a process that takes place over several months.9,23 Further, in the case of more significant trauma during large-incision anterior segment surgery that may cause a Descemet’s break, deposition of a new basement membrane also follows immediate endothelial cell migration, which can take more time, especially if the split edges are not in close proximity.9,24
Intraocular surgeries, especially involving phaco, can lead to endothelial cell damage and corneal edema, occurring in 4% to 25% of cases.25 One study concluded that, three months post-cataract surgery, average cell loss was 6.9%.26 Additionally, after initial decline, endothelial cell density continues to decrease at an annual rate of 2.5% for at least 10 years, with or without lens implant.27 Though the exact mechanism behind the increased rate of loss is unknown, theories include decreased nutrition from the aqueous humor, reduced innervation, subclinical inflammation and exposure to vitreous humor.22
Shallow anterior chamber depth, especially in the context of dense cataracts, is correlated with increased endothelial cell loss following phacoemulsification.28 Due to the confinement of space within a narrow chamber, surgeons must be cognizant of cataract density, surgery and phaco time, and ultrasound power to ensure post-op success.
During the procedure itself, it is imperative to additionally monitor for intraocular lens (IOL) contact, instrument-related trauma, incision size, irrigation solution turbulence, IOL type and ophthalmic viscoelastic substance to decrease the risk of resultant corneal edema.28,29 Endothelial cell density and morphology has been demonstrated to decrease more so with anterior chamber than posterior chamber IOLs.30 Finally, angle-supported anterior chamber intraocular lens designs can increase the risk of bullous keratopathy up to 10%, which is thought to be due to chronic inflammation from lens-endothelial cell contact at the corneal periphery.31
Loss of endothelial cell density of donor corneas has been observed following penetrating keratoplasty, which is strongly dependent upon duration of donor corneal storage and surgical trauma.22 A three-year post-op analysis indicated that cell density reaches 53% of the pre-op level.22 To cover the area of the wound, endothelial cells migrate from the recipient to the donor and from the donor to the recipient cornea.22 Finally, greater morphological abnormalities and a longer recovery time has been noted in diabetic corneas as they are considered more vulnerable to stress and trauma after cataract surgery compared with non-diabetic corneas.32
Endothelial dystrophies. Optometrists should consider the following endothelial corneal dystrophies: Fuchs’ dystrophy, posterior polymorphous dystrophy (PPD) and congenital hereditary endothelial dystrophy (CHED), with Fuchs’ being the most common.22 Fuchs’ is a bilateral disease that typically manifests in middle age or later and is marked by the presence of guttae. Although decreased cell density, polymegethism and pleomorphism are all part of both normal aging and the pathophysiology of Fuchs’, the enlarged endothelial cells in Fuchs’ secrete an excess of Descemet’s membrane in the form of banded collagen, which is not seen in senescence.22 This abnormal deposition is clinically witnessed as mushroom-like projections on the posterior surface of Descemet’s membrane; guttae can also be seen on AS-OCT and ultra-high resolution AS-OCT as distinct hyperreflective entities projecting into the aqueous.18
Guttae are not pathognomonic for Fuchs’. When they are found peripherally, they are defined as involutional Hassall-Henle bodies. In the case of Fuchs’, the guttae are found centrally. Along with guttae, folds in Descemet’s, stromal edema and microcystic epithelial edema can be seen in advanced disease.1 As the disease progresses, the natural ability of the endothelium to maintain appropriate corneal hydration is compromised, exacerbating symptoms of blur and glare upon awakening.1 Ultimately, this may necessitate keratoplasty. Interestingly, the micron-scale dimensions of guttae can affect the migratory behavior of endothelial cells.20
PPD is along the spectrum of inherited bilateral endothelial disorders in which the majority of patients have subtle clinical signs and unaffected vision. Histologically, the endothelium takes on epithelium-like characteristics. Should these cells infiltrate the trabecular meshwork, IOP may rise.1,22 Features of PPD can include vesicular, curvilinear and placoid irregularities on slit lamp exam, similar to those seen on the epithelium in basement membrane dystrophy. Rounded, dark areas with central cell detail appearing as a doughnut-like pattern on specular microscopy can also occur. In visually significant cases, the reduction is often due to edema.1
Whereas Fuchs’ and PPD typically arise beyond the fifth decade of life, CHED is present at birth, appearing as bilateral corneal clouding.22 It often requires keratoplasty, as corneal thickness can be two to three times higher than normal, and up to 1mm centrally. However, in contrast with iridocorneal endothelial syndrome, corneal diameter and IOP are within normal ranges.1 Other features of CHED can include discrete white dots in the stroma, pigskin-like roughness to the epithelial surface and peau d’orange appearance to Descemet’s.1
Contact lens-induced endotheliopathy. A metabolic source of stress, such as hypoxia from contact lens wear, can also affect the endothelium. In order for this to happen, the inciting agent must typically occur over a large span of time. Hypoxic stress can cause morphological changes to cell size and shape, alteration of microanatomy and dysfunction of the endothelium.5,22
Glaucoma-induced epitheliopathy. Changes to corneal endothelial cell density have been documented in primary open-angle glaucoma (POAG). Studies have shown patients with POAG have significantly lower endothelial cell density than age-matched controls.33 The mechanism of damage is thought to be from high IOP hindering normal metabolic function and damaging the physical barrier function of endothelial cells, similar to damage of the retinal nerve fibers in the posterior segment.33 Another theory is benzalkonium chloride preservative toxicity to the endothelium from topical hypotensive agents often used in glaucoma.33 These adverse effects to the cells appear to be dose- and time-dependent.34
Carbonic anhydrase inhibitors. Given that carbonic anhydrase is one of the key components of endothelial pumps, it has been postulated that the use of carbonic anhydrase inhibitors (CAIs) may cause disequilibrium of this system. However, studies indicate that topical use of a CAI, such as brinzolamide, has no influence on endothelial cell characteristics as examined with specular microscopy.35 Others have suggested that use of dorzolamide, for example, in patients with pre-existing endothelial cell pathology caused a 12µm increase in size. This change, however, is not considered clinically relevant.36
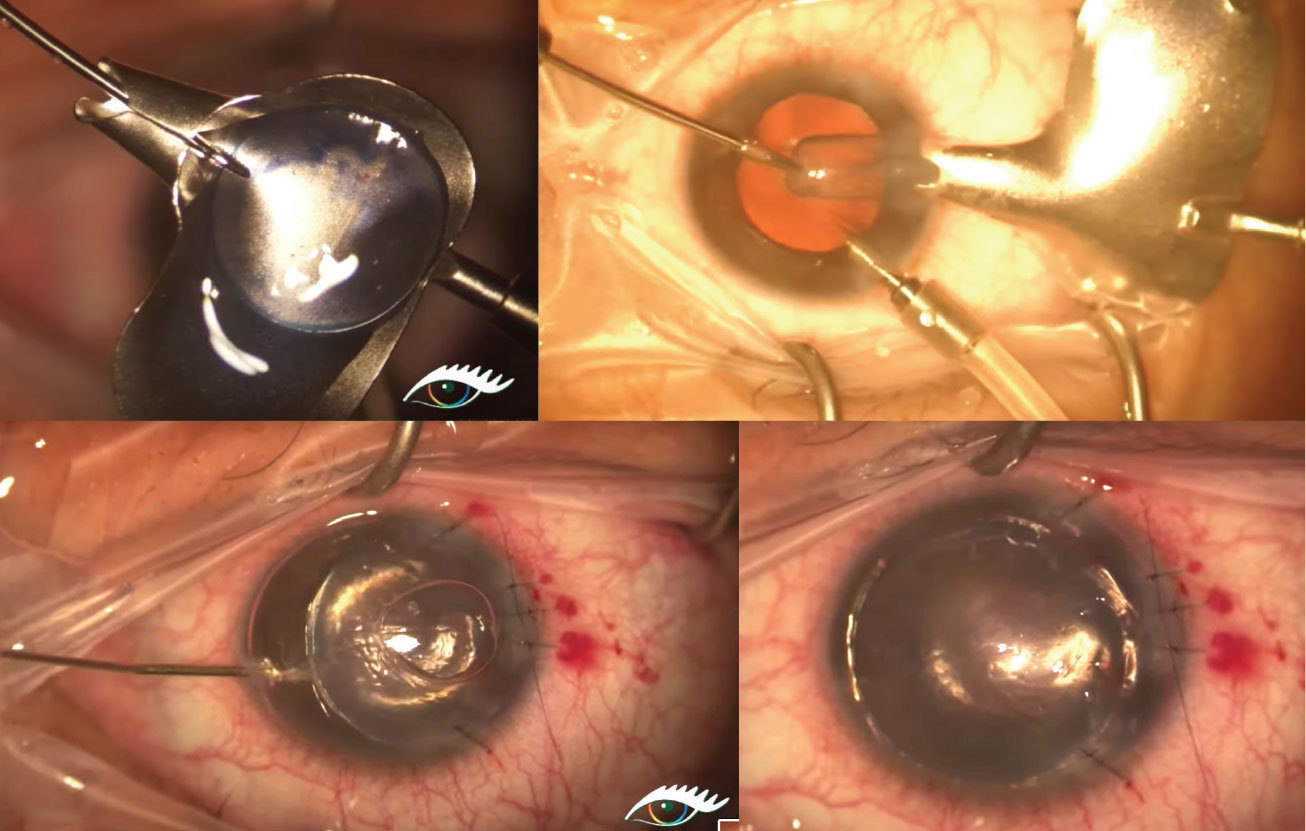 |
| Fig. 4. Top left: A 9.0mm donor graft is loaded into the plate of the glide with the endothelium side up. Top right: The donor tissue is folded up like a taco and pulled through the funnel-shaped portion of the glide, then pulled through the temporal incision. Bottom left: After the graft has been successfully inserted into the anterior chamber, it is unfolded in the recipient anterior chamber with balanced saline solution and/or an air bubble. Bottom right: The donor button maintains apposition with the host cornea through use of an air bubble, giving time for the endothelial pumps to aid in donor-host tissue binding. Photo: James Lewis, MD. Click image to enlarge. |
Interventions
The current standard of care for advanced endothelial compromise is surgery, typically DSEK (or the automated version, Descemet’s stripping automated endothelial keratoplasty—DSAEK) and DMEK. While DSEK/DSAEK and DMEK both provide a reprieve for endothelial decompensation, DSEK/DSAEK involve descemetorhexis of the host’s cornea and grafting with donor tissue that consists of endothelium, Descemet’s and some stromal tissue (Figure 4). By contrast, DMEK has a thinner donor graft by eliminating stromal tissue completely. Penetrating keratoplasty is often reserved for patients with severe end-stage edema or deep scarring of the stroma, in which a full-thickness graft is necessary.31
DMEK is growing in popularity compared with DSEK, providing rapid, predictable and efficacious visual rehabilitation for endothelial decompensation with minimal changes to refractive error.31 Though descemetorhexis without grafting is also an option, it is no the standard of care for all endothelial compromise as it requires careful patient selection, limiting research potential in large-scale studies.
Though keratoplasties are the standard surgical treatment for corneal decompensation, donor corneas can be difficult to come by. Alternative ways to improve vision and decrease pain/discomfort include topical osmotic solutions, bandage contact lenses (alone or in combination with hypertonics), amniotic membranes, stromal puncture or phototherapeutic keratectomy for advanced cases of epithelial compromise from persistent edema or a Gundersen conjunctival flap to reduce pain in severe cases of bullous keratopathy.31
There is excitement in the research community over approaches that may preclude or delay surgery. This includes collagen crosslinking or topical rho-associated kinase (ROCK) inhibitors. Although crosslinking is traditionally seen as a treatment for strengthening stromal collagen fibers in ectasia, transendothelial inflow and stromal imbibition pressure have been shown to decrease. This leads to resolution of bullae and improved vision and symptoms following the procedure; however, it remains experimental to date.37 ROCK inhibitors promote endothelial cell proliferation, enhance cell adhesion and suppress apoptosis on endothelial cells in vitro.38 ROCK inhibitors applied topically to edematous corneas after cataract surgery have proven beneficial in improving corneal clarity.39 As such, both in vivo and ex vivo use of ROCK inhibitors that proliferate cultured cells may be useful for endothelial compromise.31
The future of corneal endothelial management may lie in the use of culturing and proliferating endothelial cells, as well as pluripotent or multipotent stem cells, to produce additional endothelial cells in bioengineering corneal endothelium.40 Regeneration of healthy corneal endothelium via the use of transplanting cultured endothelial cells has been established with culture protocols and transplantation techniques that are currently under investigation.41 Various techniques that may even aim for multiple cells from a single donor cornea to be used in hundreds of patients are also under investigation.40 Finally, many pathologies of the endothelium leave the peripheral tissue unaffected. Given the migratory potential of endothelial cells, investigation of regeneration in the absence of implantation of donor tissue is also being considered.40
Dr. Minhas is director of on-campus residency programs and an assistant professor at Salus University/Pennsylvania College of Optometry.
| 1. Rosado-Adames N, Afshari NA. “Corneal Endothelium.” Yannoff M. Ophthalmology, 4th edition. Saunders. 2013;264-268. 2. Kocluk Y, Burcu A, Sukgen EA. Demonstration of cornea Dua’s layer at a deep anterior lamellar keratoplasty surgery. Oman J Ophthalmol. 2016;9(3):179-81. 3. Dua HS, Said DG. Clinical evidence of the pre-Descemets layer (Dua’s layer) in corneal pathology. Eye (Lond). 2016;30(8):1144-5. 4. Bonanno JA. Molecular mechanisms underlying the corneal endothelial pump. Exp Eye Res. 2012; 95(1):2-7. 5. Zhiguo H, Forest F, Gain P, et al. 3D map of the human corneal endothelial cell. Sci Rep 6. 2016; https://doi.org/10.1038/srep29047. 6. Abib FC, Hida RY, dos Santos RM. Corneal endothelium: histology, physiology and In-vivo examination with specular microscope. JSM Ophthalmol. 2017;5(4):1063. 7. Claerhout I, Beele H, Kestelyn P. Graft failure: I. endothelial cell loss. Int Ophthalmol. 2008;28(3):165-73. 8. Murphy C, Alvarado K, Juster R, et al. Prenatal and postnatal cellularity of the human corneal endothelium. A quantitative histologic study. Invest Ophthalmol Vis Sci. 1984;25(3):312-22. 9. DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37(3):588-98. 10. Polse KA, Brand RJ, Cohen SR, et al. Hypoxic effects on corneal morphology and function. Invest Ophthalmol Vis Sci. 1990;31(8):1542-54. 11. Toslak D, Thapa D, Erol MK, et al. Smartphone-based imaging of the corneal endothelium at sub-cellular resolution. J Mod Opt. 2017; 64(12): 1229-32. 12. Fliotsos M, Deljookorani S, Dzhaber D, et al. Qualitative and quantitative analysis of the corneal endothelium with smartphone specular microscopy. Cornea. 2020;39(7):924-9. 13. Kopplin LJ, Przepyszny K, Schmotzer B, et al. Relationship of Fuchs’ endothelial corneal dystrophy severity to central corneal thickness. Arch Ophthalmol. 2012;130(4):433-9. 14. Tananuvat N, Khumchoo N. Corneal thickness and endothelial morphology in Normal Thai eyes. BMC Ophthalmol. 2020;20(1):167. 15. Prasad A, Fry K, Hersh PS. Relationship of age and refraction to central corneal thickness. Cornea. 2011;30(5):553-5. 16. Amiri MA, Hashemi H, Ramin S, et al. Corneal thickness measurements with Scheimpflug and slit scanning imaging techniques in keratoconus. J Curr Ophthalmol. 2017;29(1):23-7. 17. Han SB, Liu YC, Noriega KM, et al. Applications of anterior segment optical coherence tomography in cornea and ocular surface diseases. J Ophthalmol. 2016;2016: 4971572. 18. Shousha MA, Perez VL, Wang J, et al. Use of ultra-high resolution optical coherence tomography to detect in vivo characteristics of Descemet’s membrane in Fuchs’ dystrophy. Ophthalmology. 2010;117(6):1220-7. 19. Jiao H, Hill LJ, Downie LE, et al. Anterior segment optical coherence tomography: its application in clinical practice and experimental models of disease. Clin Exp Optom. 2019;102(3):208-17. 20. Ali M, Raghunathan V, Li JY, et al. Biomechanical relationships between the corneal endothelium and Descemet’s membrane. Exp Eye Res. 2016;152:57-70. 21. Abib FC, Barreto J. Behavior of corneal endothelial density over a lifetime. J Cataract Refract Surg. 2001;27(10):1574-8. 22. Bourne WM. Biology of the corneal endothelium in health and disease. Eye. 2003;17: 912-8. 23. Watsky MA, McDermott ML, Edelhauser HF. In vitro corneal endothelial permeability in rabbit and human: the effects of age, cataract surgery and diabetes. Exp Eye Res. 1989;49(5):751-67. 24. Kim T, Hasan SA. A new technique for repairing Descemet membrane detachments using intracameral gas injection. Arch Ophthalmol. 2002;120(2):181-3. 25. Ravalico G, Tognetto D, Palomba MA, et al. Corneal endothelial function after extracapsular cataract extraction and phacoemulsification. J Cataract Refract Surg. 1997;23(7):1000-5. 26. Reuschel A, Bogatsch H, Barth T, et al. Comparison of endothelial changes and power settings between torsional and longitudinal phacoemulsification. J Cataract Refract Surg. 2010;36(11):1855-61. 27. Bourne WM, Nelson LR, Hodge DO. Continued endothelial cell loss ten years after lens implantation. Ophthalmology. 1994;101(5):1014-23. 28. Hwang HB, Lyu B, Yim HB, et al. Endothelial cell loss after phacoemulsification according to different anterior chamber depths. J Ophthalmol. 2015;2015:210716. 29. Storr-Paulsen A, Norregaard JC, Farik G, et al. The influence of viscoelastic substances on the corneal endothelial cell population during cataract surgery: a prospective study of cohesive and dispersive viscoelastics. Acta Ophthalmol Scand. 2007;85(2):183-7. 30. Numa A, Nakamura J, Takashima M, et al. Long-term corneal endothelial changes after intraocular lens implantation. Anterior vs posterior chamber lenses. Jpn J Ophthalmol. 1993;37(1):78-87. 31. Feizi S. Corneal endothelial cell dysfunction: etiologies and management. Ther Adv Ophthalmol. 2018;10:2515841418815802. 32. Tang Y, Chen X, Zhang X, et al. Clinical evaluation of corneal changes after phacoemulsification in diabetic and non-diabetic cataract patients, a systematic review and meta-analysis. Sci Rep 7; 2017:14128. 33. Yu AY, Wu L, Qu B. Changes in corneal endothelial cell density in patients with primary open-angle glaucoma. World J Clin Cases. 2019;7(15):1978-85. 34. Chen W, Li Z, Hu J, et al. Corneal alternations induced by topical application of benzalkonium chloride in rabbit. PLoS One. 2011;6(10):e26103. 35. Nakano T, Inoue R, Kimura T, et al. Effects of brinzolamide, a topical carbonic anhydrase inhibitor, on corneal endothelial cells. Adv Ther. 2016;33(8):1452-9. 36. Wirtitsch MG, Findl O, Kiss B, et al. Short-term effect of dorzolamide hydrochloride on central corneal thickness in humans with cornea guttata. Arch Ophthalmol. 2003;121(5): 621-5. 37. Ehlers N, Hjortdal J. Riboflavin–ultraviolet light induced cross-linking in endothelial decompensation. Acta Ophthalmol. 2008;86(5):549-51. 38. Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50(8):3680-7. 39. Okumura N, Inoue R, Okazaki Y, et al. Effect of the rho kinase inhibitor Y-27632 on corneal endothelial wound healing. Invest Ophthalmol Vis Sci. 2015;56(10):6067-74. 40. Price MO, Mehta JS, Jurkunas UV, et al. Corneal endothelial dysfunction: evolving understanding and treatment options. Prog Retin Eye Res. 2020:100904. 41. Okumura N, Koizumi N. Regeneration of the corneal endothelium. Curr Eye Res. 2020;45(3):303-12. |


