Antimicrobials are an exemplary display of therapeutic evolution. The historical medicine cabinet of antimicrobials has been stocked with increasingly efficacious therapies that have been incredibly powerful and fast acting. However, time has not been a friend to this class of drugs. Co-evolving antibacterial resistance is the main enemy, and the current pipeline of antimicrobial therapies must adapt to respond to this omnipresent threat.
In this article, we will discuss clinical research and new antimicrobial therapies in development that show promise in treating today’s—and tomorrow’s—ocular infections.
The Origin of Species
The problem with any antibacterial medication is that the battle must be fought continuously. An antibiotic may work effectively initially, but over time, ocular microbes can become resistant.
Upon exposure to an antibacterial agent, bacteria that are naturally resistant to a drug have a chance to proliferate preferentially over more susceptible bacteria. We commonly––and incorrectly––believe that bacteria are “developing”increased resistance to a certain drug; in actuality, the proportion of bacterial isolates naturally resistant to that drug is increasing. Remember, bacteria do not become resistant to an antibiotic after exposure. Rather, a genetic mutation in the encoding alters their susceptibility to the drug. Upon exposure to an antibacterial agent, bacteria with these types of natural resistance mutations will be able to reproduce at a quicker rate than the vulnerable bacteria. Over time, the bacterial population shifts to contain a greater proportion of antibacterial-resistant bacteria.
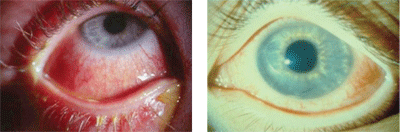
New innovations in antimicrobial therapy aim to target such as infections bacterial conjunctivitis (left) and viral conjunctivitis (right).
Keep in mind, though, that bacteria can resist drugs through one or more potential mechanisms. Resistance to penicillin and cephalosporins can be achieved by bacterial expression of a beta-lactamase enzyme, which inactivates the molecular structure of the drug. In other cases, mutations of their cell wall receptors reduce or block the drug’s ability to bind to the bacteria. Bacteria with mutated DNA gyrase or topoisomerase IV enzymes can evade fluoroquinolone antibacterial actions.1
There is a large genetic variation between bacterial species; because of this, it’s likely that some bacteria will be naturally resistant to any given antibacterial.
Antibiotics and Beyond
Besides bacterial conjunctivitis and blepharitis, sight-threatening ocular infections (e.g., microbial keratitis and endophthalmitis) are the most serious diseases that antibiotics target. Although many effective ophthalmic antibacterials are available in the United States today, broad-spectrum fluoroquinolones constitute a majority of the market, as they have been shown to be a valuable addition to the anti-infective spectrum. However, they face progressively higher levels of resistance. Susceptibility surveys such as the Ocular TRUST (Tracking Resistance in U.S. Today) longitudinal surveillance study, which examined the in vitro antimicrobial susceptibility of ocular isolates collected in the U.S. from 1999 and 2006, revealed an increasing incidence of multidrug-resistant organisms in ocular infections. The study also found that the prevalence of methicillin resistance among the isolates increased from 29.5% in 2000 to 41.6% in 2005.2
It has also been reported that methicillin-resistant Staphylococcus aureus (MRSA) infections account for 18% of culture-positive cases of endophthalmitis.3 Moreover, methicillin resistance shares certain links with fluoroquinolone resistance: If a bacterium is methicillin resistant, there is an 85% chance that it will also be resistant to the entire class of fluoroquinolones.2
The Antibiotic Resistance in Ocular Microorganisms (ARMOR) surveillance study found a high level of MRSA strains, and the Ocular TRUST study confirmed the high concordance between methicillin resistance and pan-fluoroquinolone resistance in Staphylococcus aureus and Staphylococcus epidermidis.1,4
In an effort to combat this resistance concern, promising new drugs in the quinolone family—namely, isothiazoquinolones (ITQs)—are in development and add a third mechanism of action as potent DNA primase inhibitors.5-10 Fluoroquinolones typically inhibit both DNA gyrase and topoisomerase IV, which are required for the DNA replication process. This new class has been found to have good in vitro and in vivo activities against several key bacterial pathogens such as Staphyloccocus aureus, including MRSA isolates.5
ITQs are structurally different from fluoroquinolones, with substitution in the typical 3-carboxyl group by an isothiazolone ring. This modification helps with inhibition of DNA primase, thereby increasing efficacy and decreasing the chances of developing resistance compared with fluoroquinolones. Bacterial DNA primase is essential for DNA replication in gram-positive and gram-negative bacteria, and is also structurally distinct from eukaryotic primases. It represents an attractive target for therapeutic intervention. With that in mind, in a survey designed to acess ocular pathogen prevalence and emerging antibiotic therapy, researchers examined the in vitro activity of a novel ITQ, ACH-0139586, against ocular pathogens (S. aureus, S. epidermidis, S. pneumoniae, H. influenza, M. catarrhalis and P. aeruginosa) compared with moxifloxacin and gatifloxacin. The study demonstrated that ACH-0139586 was more potent relative to gatifloxacin and moxifloxacin, regardless of methicillin and fluoroquinolone resistance, and that this was most apparent against evaluated gram-positive pathogens.
ACH-0139586 has a novel target profile against bacterial DNA replication enzymes and potent broad-spectrum bactericidal activity, characteristics that indicate it may play an important role against drug-resistant bacteria.11 Because of its exciting antibacterial spectrum and positive kill curves, this novel compound shows great promise as a next generation treatment.
The Need for New Antivirals
On the other side of the anti-infective spectrum, advances in antiviral therapy are slowly making their way to the forefront of ocular research and development. Treatment for viral conjunctivitis has typically been limited to symptomatic therapy and epidemiological measures of control in order to reduce transmission; or to topical corticosteroid treatment in order to reduce immune infiltration.12 A variety of viruses can be responsible for conjunctival infection; however, adenovirus is the most common cause. The competition for the first FDA-approved drug for the treatment of viral conjunctivitis is fierce. Thankfully, an array of drugs are in the pipeline for this unmet need.
NovaBay has developed an eye drop formulation for the treatment of viral conjunctivitis. NVC-422 uses NovaBay’s class of aganocides, which are topical compounds with a broad spectrum of activity against bacteria, viruses and fungi. Bacteria or viruses will be less likely to develop resistance to aganocides, a critical characteristic for antibiotics in today’s environment. Because of its broad spectrum of activity, a highlight of NVC-422 is that the formulation may prove to be useful in treating bacterial conjunctivitis as well. Aganocides demonstrated high efficacy in vitro against multi-drug resistant bacteria, including MRSA and vancomycin-resistant Enterococcus.13 NovaBay expects to complete its 450-patient, phase IIb trial in the first half of 2013.14
In addition, the Portuguese pharmaceutical company Adapt Produtos Oftalmológicos Ltda. is evaluating the efficacy and tolerability of gancliclovir ophthalmic gel 0.3% for the treatment of adenoviral conjunctivitis compared to placebo in an ongoing Phase III trial.15
Antiseptic Options
Antiseptics are used for surgical sterilization, treatment of infection, prophylaxis, and medication preservation. Endophthalmitis arising from cataract surgery is a rare but serious complication thought to derive largely from microflora in the ocular tear film, lids, and adnexa gaining entry to the anterior chamber during surgery.16 Antiseptic biocides, such as povidone-iodine, may be an effective approach to anti-infective therapy. Antiseptics contain certain advantages because of their physicochemical mechanism of action, which includes their ability to act across broad swaths of pathogens, including strains of conjunctivitis caused by adenovirus.17 Povidone-iodine is a broad-spectrum antiseptic that works by iodination of lipids and oxidation of cytoplasmic and membrane components; this chemically-based antimicrobial activity has little risk of microbial resistance, crossover capabilities, and wide applications with high degrees of efficacy.
FST-100 (povidone-iodine/dexamethasone ophthalmic suspension, Foresight Biotherapeutics) is a combination drug that focuses on microbial eradication and reducing infection-related inflammation. As compared to cidofovir and tobramycin/dexamethasone, FST-100 showed superior clinical effectiveness and virucidal activity in a rabbit model.18 Two randomized, double masked, multi-center studies are currently underway to test the safety and efficacy of FST-100 for the treatment of acute viral conjunctivitis.
Treatments and Future Therapies
Based on the pathogens involved, therapeutic agents may have a different impact on each infection. Even broad-spectrum antibacterials cannot eliminate all potential ocular pathogens. It would, therefore, be wise for clinicians to understand the difference between gram-positive and gram-negative bacteria and which antibacterial therapies are more effective against each type. The outermost cell component of gram-positive bacteria is a thick, rigid layer of peptidoglycan, and the exterior of gram-negative bacteria includes an additional liposaccharide layer.
To preserve efficacy, clinicians must employ antibiotic therapy carefully. Some strategies to slow the progress of resistance include cycling drugs, selectively using anti-infectives and combining different antibiotics. In one review, three out of four studies demonstrated that cycling reduced the rate of resistance to the class not in use.19
These new advances in ocular antimicrobial drugs show a leap toward innovation that we have not previously seen within this realm of therapy. Rather than bringing out the “new and improved” beta-lactam or macrolide, drug developers have emphasized an ever-diversifying array of antibacterial mechanisms of action. Fighting the good fight against bacterial resistance is not a battle that is easily won, but we are pushing towards the finish line for a Herculean, non-resistant drug.
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at the Schepens Eye Research Institute. Mr. Shapiro is vice president of anti-infectives and anti-inflammatories at Ora, Inc. Ms. Tobey is a medical writer at Ora, Inc.
1. Cambau E, Matrat S, Pan XS, et al. Target specificity of the new fluoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother. 2009 Mar;63(3):443-50.
2. Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008 Jun;145(6):951-8.
3. Deramo VA, Lai JC, Winokur J, et al. Visual outcome and bacterial sensitivity after methicillin-resistant Staphylococcus aureus-associated acute endophthalmitis. Am J Ophthalmol. 2008 Mar;145(3):413-7.
4. IMS National Sales Perspectives. IMS Health. 2008.
5. Pucci MJ, Podos SD, Thanassi JA, et al. In vitro and in vivo profiles of ACH-702, an isothiazoloquinolone, against bacterial pathogens. Antimicrob Agents Chemother. 2011 Jun;55(6):2860-71.
6. Agarwal A, Louise-May S, Thanassi JA, et al. Small molecule inhibitors of E. coli primase, a novel bacterial target. Bioorg Med Chem Lett. 2007 May 15;17(10):2807-10.
7. Cheng J, Thanassi JA, Thoma CL, et al. Dual targeting of DNA gyrase and topoisomerase IV: target interactions of heteroaryl isothiazolones in Staphylococcus aureus. Antimicrob Agents Chemother. 2007 Jul;51(7):2445-53.
8. Molina-Torres CA, Ocampo-Candiani J, Rendon A, et al. In vitro activity of a new isothiazoloquinolone, ACH-702, against Mycobacterium tuberculosis and other mycobacteria. Antimicrob Agents Chemother. 2010 May;54(5):2188-90.
9. Vera-Cabrera L, Campos-Rivera MP, Escalante-Fuentes WG, et al. In vitro activity of ACH-702, a new isothiazoloquinolone, against Nocardia brasiliensis compared with econazole and the carbapenems imipenem and meropenem alone or in combination with clavulanic acid. Antimicrob Agents Chemother. 2010 May;54(5):2191-3.
10 Zhang MQ, Haemers A. Quinolone antimicrobial agents: structure-activity relationships. Pharmazie. 1991 Oct;46(10):687-700.
11. Shapiro A. In vitro activity of ACH-0139586, a novel isothiazoquinolone, moxifloxacin and gatifloxacin against clinical isolates, including methicillin and fluoroquinolone resistant. Invest Ophthalmol Vis Sci. 2012;53 ARVO e-abstract 6259.
12. Kinchington PR, Romanowski EG, Jerold Gordon Y. Prospects for adenovirus antivirals. J Antimicrob Chemother. 2005 Apr;55(4):424-9.
13. D’Lima L, Friedman L, Wang L, et al. No decrease in susceptibility to NVC-422 in multiple-passage studies with methicillin-resistant Staphylococcus aureus, S. aureus, Pseudomonas aeruginosa, and Escherichiacoli. Antimicrob Agents Chemother. 2012 May;56(5):2753-5.
14. NovaBay Pharmaceuticals (NBY) receives $2.6 million from partner Galderma. NovaBay. 2012 Sep 27. Available at: www.novabaypharma.com/investors/release/NovaBay-Pharmaceuticals-Receives-2-6-Million-from-Partner-Galderma. Accessed October 2, 2012.
15. Adapt Produtos Oftalmológicos Ltda. Evaluate the use of ganciclovir gel 0.3% for the treatment of conjunctivitis caused by adenovirus. Clinical Trials. Available at:
http://clinicaltrials.gov/ct2/show/NCT01600365. Accessed October 8, 2012.
16. Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002 Jan;109(1):13-24.
17. Monnerat N, Bossart W, Thiel MA. Povidone-iodine for treatment of adenoviral conjunctivitis: an in vitro study. Klin Monbl Augenheilkd. 2006 May;223(5):349-52.
18. Hill JM. Topical FST100 dexamethasone 0.1% containing povidone-iodine 0.4% reduced the clinical signs and infectious viral titers in a rabbit model of adenoviral conjunctivitis. Poster presentation at the annual Association for Research in Vision and Ophthalmology meeting, May 2-6, 2010; Ft. Lauderdale, Fla.
19. Brown EM, Nathwani D. Antibiotic cycling or rotation: a systematic review of the evidence of efficacy. J Antimicrob Chemother. 2005 Jan;55(1):6-9.


