 |
A 25-year-old established patient called due to new onset blurry vision in the left eye. He had long-standing keratoconus (KC) and wore sclerals. He denied sleeping in lenses, wearing old ones or any other acute change to normal wear. He reported his eye was slightly red, irritated and experienced mild light sensitivity; he denied any discharge. The patient was triaged, told to remove his lens and asked to come in for a visit. Based on his symptoms, my top three working differentials were infectious keratitis, acute corneal hydrops and uveitis.
Case
In office, his ocular history included advanced KC and a history of wearing rigid gas permeable lenses, more recently switched out for sclerals in both eyes. The patient suffered from mild dry eye syndrome OU. He denied any prior ocular surgery or trauma and had no contributory medical history. He also had no noted allergies or family ocular history. Currently, he is using preservative-free artificial tears as needed in both eyes.
The patient’s entering acuity without lenses or glasses was 20/200, pinhole 20/40 OD and 20/250, pinhole 20/100 OS. Intraocular pressure (IOP) was 17mm Hg OD, 21mm Hg OS. Confrontation visual fields were full in both eyes. Pupils revealed no relative afferent pupillary defect in either eye and extraocular muscles were full. Pachymetry reading were 550µm OD, unable OS. Slit lamp examination was unremarkable OD, but a protective ptosis was revealed OS. The conjunctiva and sclera had diffuse injection OS. Corneal slit lamp exam showed inferior conical protrusion with a focal area of inferior corneal edema. Overlying microcystic edema and bullae were seen, as was an intact epithelium. No infiltrates or keratic precipitates were seen, anterior chamber was deep with no cells observed and iris architecture was normal and the lens was clear.
The patient was diagnosed with acute corneal hydrops in the setting of KC. Other differential diagnoses were infectious keratitis, autoimmune keratitis, Fuchs’ corneal endothelial dystrophy, iridocorneal endothelial syndrome, posterior polymorphous corneal dystrophy and others.
He was ordered to halt lens use temporarily and started on prednisolone QID, Muro 128 drops (sodium chloride 5%, Bausch + Lomb) QID and cyclopentolate BID; a bandage contact lens was placed to help with comfort. An IOP-lowering drop was considered due to borderline IOP from inflammation but was not prescribed. Over the next month, the patient gradually improved and so did his vision. He was left with scarring and some contour irregularity where the hydrops were, but was able to start back into scleral lenses and regain 20/40- in the affected eye.
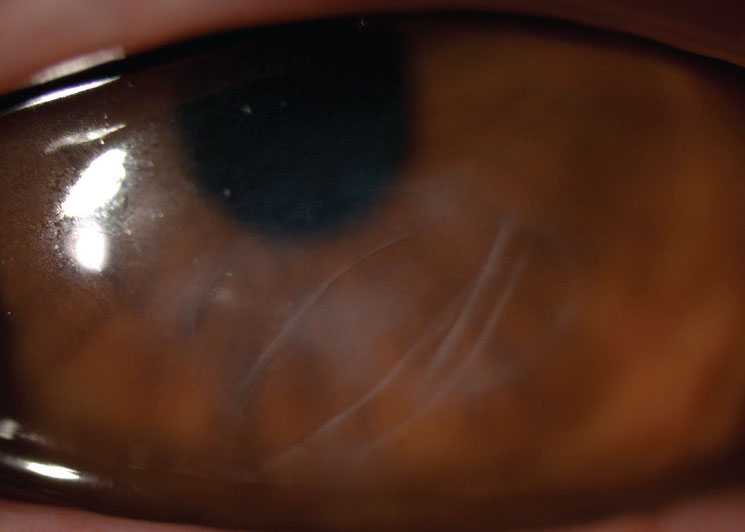 |
|
Residual scarring after corneal hydrops. Click image to enlarge. |
Corneal Hydrops
The etiology of acute corneal hydrops is a breakdown of Descemet’s membrane (DM) in the setting of advanced corneal ectasia. The rupture happens from stretching of the DM; once this occurs, aqueous enters the corneal stroma and epithelium. The term “hydrop” refers to the abnormal fluid accumulation. Acute hydrops occur in roughly 3% of KC patients. The average onset is around 25 years of age, affecting more men than women. These patients often have an associated history of eye rubbing, seasonal allergies and advanced corneal ectasia.1 The most common cause of DM detachment is not acute corneal hydrops, but an association with intraocular surgery.
When hydrops also has focal corneal edema, it is indicative of failure of the DM’s barrier function, causing fluid consumption by the overlying corneal stroma. Most cases of acute hydrops resolve on their own over two to four months. Final outcomes vary, depending on amount of swelling and time taken to resolve. Patients can be left with a range of corneal neovascularization and corneal scarring.2 Symptoms and signs to look for include corneal edema, often associated with decreased visual activity, epiphora, photophobia, injection and pain.
In office, slit lamp photos are recommended for documentation and monitoring purposes. Anterior segment OCT is also beneficial to understand the location and extent of corneal edema and the magnitude of DM breakage. The mechanism of repair for DM involves reattachment of the DM and endothelial migration. When the DM ruptures, it often coils and retracts. Therefore, the first part of healing involves the DM reattaching to the posterior stroma; this period can vary depending on the severity of the break. Then, the endothelium has to migrate to close the gap between the broken DM edges and produce a new DM.3,4
Treatment
Options for managing hydrops can range from observation to more extreme modalities. Traditional treatment involves medical therapy similar to the patient above. Hypertonic sodium chloride is prescribed to reduce epithelial edema and cycloplegia can be prescribed for patient comfort, but a cycloplegic agent is not always given. Topical steroids are controversial, thus not prescribed by all. In this case, steroids were prescribed to help reduce inflammation and hopefully prevent further neovascularization formation. The bandage contact lens was large diameter (>16mm), placed for patient comfort, but varying sizes can be used for improved relief.
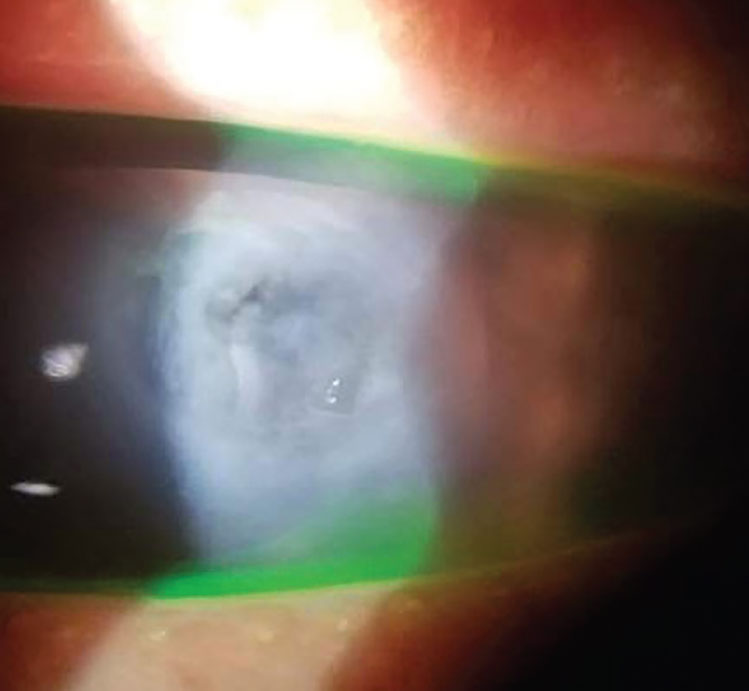 |
|
A case of an acute corneal hydrops. Photo: Steven Sorkin, OD. Click image to enlarge. |
There are alternative management options for acute hydrops that involve comanagement with an ophthalmologist. Around 15 to 20 years ago, pneumatic descemetopexy was introduced to tamponade the DM break. This procedure involves placing an air or gas bubble in the anterior chamber to plug the break; this is thought to reduce the resolution time of edema. Through quickened recovery, this likely reduces the period of discomfort and decreased vision while also reducing the risk of visually significant scarring.5 One study found corneal edema in nine patients treated with pneumatic descemetopexy lasted on average 20 days, while 21 non-treated patients had an average of 65 days until resolution. They also found those treated with the intracameral air were able to return to their hard contact lenses in a quarter of the time of the control group. Once the edema had resolved in both groups, they did not find a difference in best-corrected visual acuity.5
The injection of air or gas can reattach the DM to the posterior stroma; however, it does not aid in the migration and creation of new DM by the endothelium. Compression sutures are thought to help with reattachment and hold the edges of the tear close, which would enable endothelial cells to seal more rapidly.3 Consequently, several groups in the literature report using an air bubble in combination with sutures added through the DM to more tightly adhere to the stroma after hydrops.6
Surgeons have the choice between choosing air and gas when performing pneumatic descemetopexy, with the decision often based upon the amount of time needed to repair the defect in DM. Air lasts the least amount of time, ranging from two to three days, which can result in the patient needing a repeat bubble placement. Conversely, sulfur hexafluoride (SF₆) normally lasts around seven to 10 days, which is sufficient for healing of DM.5 To date, there has not yet been a study published finding that performing a pneumatic descemetopexy reduces the need for corneal transplantation.6
A last resort option for these patients is penetrating keratoplasty (PK). Other transplants like deep anterior lamellar keratoplasty are not commonly performed due to the difficulty of separating DM from the posterior stroma. The data for PK post-hydrops does not show a difference in graft survival because of previous episodes for acute corneal hydrops. An increase in graft rejection is believed to be due to the inflammation that usually accompanies hydrops.4
Acute corneal hydrops can be devastating to corneal ectasia patients. Identifying them and starting proper treatment can assist in a better visual outcome long-term. Often, these patients can return to their hard contact lenses with sufficient vision, but in severe cases, more aggressive treatment is necessary.
1. Fan Gaskin JC, Good WR, Jordan CA, Patel DV, McGhee CNJ. The Auckland keratoconus study: identifying predictors of acute corneal hydrops in keratoconus. Clin Exp Optom. 2013;96(2):208-13. 2. Mulhern M, Barry P, Condon P. A case of Descemet’s membrane detachment during phacoemulsificaton surgery. Br J Ophthalmol. 1996;80(2):185-6. 3. Özcan G, Uçakhan ÖÖ. Surgical management of corneal hydrops: case series. Turk J Ophthalmol. 2022;52(1):64-8. 4. Greenwald MF, Vislisel JM, Goins KM. Acute corneal hydrops. EyeRounds. eyerounds.org/cases/241-acute-corneal-hydrops.htm. August 3, 2016. Accessed September 26, 2023. 5. Miyata K, Tsuji H, Tanabe T, et al. Intracameral air injection for acute hydrops in keratoconus. Am J Ophthalmol. 2002;133(6):750-2. 6. Rajaraman R, Singh S, Raghavan A, Karkhanis A. Efficacy and safety of intracameral perfluoropropane (C3F8) tamponade and compression sutures for the management of acute corneal hydrops. Cornea. 2009;28(3):317-20. |


