Keratoconus affects approximately one in 1,800 individuals, according to an epidemiological study published in 1986.1 That statistic suggests about 150,000 keratoconic patients in the United States. From this estimate, we can deduce that the average eye care practitioner should encounter just a few keratoconus patients each year.
Yet, keratoconus seems to garner a disproportionately greater amount of coverage within continuing education lectures and trade articles. Arguably, this is because the concepts behind contact lens treatment for keratoconus are broader in reach. They apply to other corneal irregularity patients, such as post-corneal surgery (penetrating keratoplasty, failed refractive surgery, post-LASIK ectasia), scarring from corneal ulcers or trauma, or thinning disorders, such as pellucid marginal degeneration (PMD). Secondly, the management of keratoconus is challenging for both patients and practitioners alike, which warrants the additional clinical attention.
Extreme Eye Care
Keratoconus is a condition of extremes. It frequently pushes our common clinical measurements to the limit, while challenging our management plan in the same fashion.
When refracting keratoconus patients, the multifocality of the cornea often causes a variable endpoint. In normal patients, the typical just-noticeable difference during refraction is plus or minus 0.25D. But in keratoconus, especially in advanced cases, it is sometimes necessary to refract with a spherical lens change of plus or minus 1.00D to 3.00D to give a discernable difference. Furthermore, the Jackson cross cylinder (JCC) in the phoropter is often not adequate to provide a noticeable difference during refraction. One strategy is to pull out the handheld JCC marked with +/- 1.00D and hold that over the phoropter aperture or trial frame.
With manual keratometry, you’ll find some keratoconic corneas so steep that their readings are out of range with drum readings. In these cases, tape a +1.25D or even a +2.25D trial lens to the objective face of the keratometer (the side that points toward the patient). Use cellophane tape, and make sure that the trial lens handle does not obscure your ability to read the mires. To get a rough estimate of the actual K readings, add 8.00D or 16.00D to your measurements, depending on whether you’ve used a +1.25D or +2.25D trial lens, respectively.
If you are using a millimeters-of-radius-to-diopter conversion slide rule, don’t be surprised if you slide the card all the way to the end and still can’t get the needed reading because the corneas are too steep. For these less common conversions, various online keratometry converters are available.
Intraocular pressure readings in keratoconus—whether with applanation or non-contact tonometry—are often artificially low. No, these patients do not have hypotony. It’s just that their corneas are thin and weak, and this throws off the assumed corneal rigidity used by the tonometers in giving their measured IOP. In these cases, you should be on the lookout for other telltale signs of glaucoma, especially optic nerve changes.
Increasing Prevalence?
The past decade seems to have brought forth a much larger number of newly diagnosed keratoconus patients than previous decades. This is in part due to better diagnostic sensitivity, especially with the increasing use of corneal topographers in routine clinical practice. It also reflects newly identified keratoconus patients who receive their diagnoses during consultations for laser vision. Keratoconus is a widely accepted contraindication for LASIK, due to the risk of worsening patients’ corneal distortion. Still, it’s not difficult to imagine why keratoconus patients—frustrated with inadequate vision from glasses and soft contact lenses—are attracted to the prospect of laser vision correction.
Growth of the laser correction industry indirectly benefits the keratoconus community; increased screening for keratoconus prompted by consultations for laser vision correction create a larger pool of identified keratoconus patients. In turn, a growing identified number of keratoconus cases incentivizes development of alternative and improved treatment. The number of laser vision correction procedures has plummeted in recent years, mirroring the economic downturn.2 Yet, a rebound in consumer confidence will once again drive new laser vision correction consultations, along with the byproduct of new diagnoses of keratoconus.
In a retrospective analysis published in 2003 that examined 1,392 laser vision correction candidates, researchers identified 13 (0.9%) as having keratoconus, suspected keratoconus or pellucid marginal degeneration.3 This is much greater than the aforementioned prevalence within the general population. So, while the actual number of keratoconus cases is probably not increasing, improved detection and greater screening volume are leading to an increased rate of keratoconus diagnosis.
An Inflammatory Etiology?
For years, keratoconus has been accepted as a non-inflammatory corneal ectasia. But, new data suggest that there may be a low-grade inflammatory component to keratoconus.4-6 This research has found that the tears of keratoconus subjects and those with suspected keratoconus have a higher expression of inflammatory molecules, including Interleukin-6, TNF-alpha and MMP-9. Many clinicians have noted that keratoconus patients tend to have corneal vascularization with superficial pannus and also deep stromal vessels; perhaps this is another byproduct of low-grade corneal inflammation as well as contact lens overwear.7
If low-grade inflammation indeed exists with keratoconus, it begs the question whether there is a role for immunosuppressants, such as topical cyclosporine for the off-label use in minimizing advancing corneal distortion in keratoconus, especially during the adolescent years. But, its efficacy in altering keratoconus progression has not been definitively established. Certainly, many clinicians treat the atopy that commonly accompanies keratoconus with topical anti-allergy drugs, such as Patanol (olopatadine 0.1%, Alcon) or Pataday (olopatadine 0.2%, Alcon), Alrex (loteprednol, Bausch + Lomb), or various over-the-counter ketotifen eye drops.
Diagnosing Mild Keratoconus
One of the classic manifestations of advanced keratoconus is Munson’s sign, where the lower eyelid margin deflects over the cone apex. Yet, this is not useful for diagnosing mild or forme fruste keratoconus. One of the emerging diagnostic tools for mild keratoconus is wavefront aberrometry. While the older literature describes irregular astigmatism as a hallmark of keratoconus, a more up-to-date description is that keratoconus patients have an unusually elevated amount of cornea-related higher-order aberrations or refractive error that is not correctable using the lower-order components of sphere, cylinder and axis (see “Recognizing Mild Keratoconus”). In particular, keratoconus patients tend to have an elevated magnitude of the higher-order aberration coma.8,9 For a patient with pure coma, a point source of light will look like a tailed comet rather than a compact point. Elevated higher-order aberrations create greater response variability during manifest refraction and might tip off a clinician to suspect keratoconus (see “A Diagnostic Challenge").
If wavefront aberrometers significantly replace the role of autorefractors in the primary care setting, they will provide practitioners with yet another tool for detecting mild keratoconus. For example, if a patient displays an elevated coma with aberrometry, it may prompt the clinician to order a corneal topography and confirm the existence of mild keratoconus.
Recognizing Mild Keratoconus
New glasses were prescribed, and the
patient was advised of the diagnosis of mild keratoconus. Michael was
cautioned against rubbing his eyes and asked to have other family
members undergo eye examinations to rule out keratoconus. We scheduled
a one-year follow-up to examine his eyes for further corneal changes. Wavefront aberrometry demonstrated unusually elevated total higher order aberrations in the left eye.
”Michael,” a 20-year-old male, presented
for routine examination with no subjective changes in vision. His
manifest refraction was -2.00 -1.00 x 170 (20/15-) O.D. and -3.00 -0.25
x 160 (20/15-) O.S. The patient’s ocular health was unremarkable with
no observable corneal abnormalities. Wavefront aberrometry (ZView,
Ophthonix) showed a notable asymmetry in total higher-order
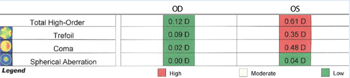
aberrations—0.12D O.D. and 0.61D O.S. In particular, the left eye
showed an unusually high amount of coma (0.48D). Manual keratometry
showed grade 1 mire distortion in the left eye. These results prompted
us to order corneal topography, which confirmed central steepening in
the left eye.
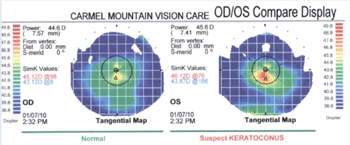
Corneal topography confirmed central steepening in the left eye.
Collagen Cross-Linking
Collagen cross-linking (CXL) is an investigational procedure that is suggested to halt keratoconic progression.10,11 Riboflavin drops are applied to the surgically de-epithelialized cornea, which is then exposed to ultraviolet A light for 30 minutes. The result: an increased biomechanical stiffness of the cornea and reduced corneal curvature, due to riboflavin/UVA-induced cross-linking of the corneal collagen. Biomechanical measurements have indicated that cross-linking can increase human corneal rigidity by over 300%.12
While the results to date are encouraging, rare complications include corneal melt with perforation and permanent corneal haze.13,14 Another study reported four cases of keratitis and corneal scarring from a total of 117 keratoconic eyes treated with CXL.15
In January 2008, it was announced that the U.S. Food and Drug Administration permitted randomized, controlled clinical trials to evaluate the safety and efficacy of CXL for progressive keratoconus and corneal ectasia.16 Interim data of the first trial, presented at the American Society of Cataract and Refractive Surgery (ASCRS) Meeting in April 2009, showed impressive results. With 457 eyes treated with CXL, the procedure indeed appeared to decrease corneal curvature while increasing corneal rigidity.17 Complications appeared to be few, including four cases of infiltrates (0.8%), four cases of delayed re-epithelialization and one case of uveitis, which may not have been related to the treatment.17
Corneal Keratoplasty: Lamellar and Full-Thickness
Penetrating keratoplasty is regarded as the end-of-the-line treatment for keratoconus. One study published in 1994 found that 22% of keratoconic patients required a penetrating keratoplasty to rehabilitate vision.18 With improved contact lens treatment and surgical alternatives including Intacs (Addition Technology) and CXL, however, it is likely that the actual number of keratoconus patients undergoing penetrating keratoplasty is significantly lower today. Still, corneal transplantation is indicated when corneal distortion, tensile weakening and progressive thinning (leading to visual fluctuations) and scarring in the visual axis are too severe for any other treatment to restore good vision. Overall, penetrating keratoplasty is a highly successful surgery; about 95% of patients achieve an optically clear donor cornea. Still, most will require post-operative refractive correction—up to 30% require spectacles and 47% require contact lenses afterwards.19
In recent years, some corneal surgeons prefer new lamellar keratoplasty to traditional penetrating keratoplasty (PK). Deep anterior lamellar keratoplasty (DALK) entails using filtered air to separate the underlying Descemet’s membrane and endothelium from the corneal stroma, called the Anwar “big bubble” technique. The advantages of DALK include avoidance of intraocular surgical risks, preservation of the host endothelium and the option of possible PK later if needed.20 One retrospective cohort study compared outcomes of DALK and PK and found that the DALK group had a significantly lower incidence of post-operative complications compared with PK cases, including allograft rejection and glaucoma.21 Another study, however, found a similar rate of complications between DALK and PK.22 (DALK is regarded as a more technically difficult surgery to perform.23)
IntraLase-enabled keratoplasty (IEK) is another alternative to traditional trefine penetrating keratoplasty. The IntraLase femtosecond laser (Abbott Medical Optics) is used instead of the trephine to shape the edge of the corneal button with complementary shapes “sculpted” into both the host and donor edge. The broader wound area leads to increased strength after healing, lessened astigmatism and perhaps even a faster recovery.
It is clear that traditional PK is under assault for the surgical treatment of choice in keratoconus. But, it’s currently difficult to foresee which of these two procedures, DALK or IEK, or yet another procedure under development, will prove to be most efficacious for treating keratoconus.
A Diagnostic Challenge
One week later, she returned still
complaining of poor vision in the left eye with the new glasses. The
prescription was verified, but visual acuity in the left eye was 20/30.
Repeated refraction was +6.25 -2.50 x 084 O.S., yielding 20/20-visual
acuity. The refraction endpoint was variable, with a just-noticeable
difference of approximately +/- 0.75D. The variable refraction
endpoint, reduced best-spectacle corrected visual acuity and
against-the-rule astigmatism were collectively suspicious for forme
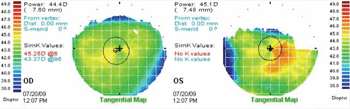
fruste keratoconus. Corneal topography was ordered and confirmed the
presence of irregular steepening in the left eye. The patient was advised of the diagnosis and presented with the contact lens option, but she elected to stay with spectacles. This case illustrates how the multifocal
nature of the keratoconic cornea can cause variable vision and a
multimodal refraction endpoint. Given this patient’s age, however,
progression (especially to the point of requiring corneal surgery) is
unlikely without other factors, such as refractive surgery. A variable refraction in the left eye prompted corneal topography, which confirmed asymmetric corneal steepening in that eye.
Intacs for Keratoconus
”Florence,” a 78-year-old female,
presented for routine examination with a complaint of poor vision in
her left eye with existing glasses. Her presenting acuity was +3.50
-2.00 x 097 (20/20) O.D., and +3.50 -2.25 x 074 (20/40-) O.S. Her
manifest refraction was +3.75 -2.25 x 098 (20/20+) O.D., and +5.25
-2.50 x 084 (20/20+) O.S. Florence’s ocular health findings were
unremarkable, and her corneas showed no abnormalities. New glasses with
the manifest refraction were prescribed.
Intracorneal ring implantation (Intacs) for keratoconus was first performed in 1997 in France.24 Since then, Intacs have become accepted as a surgical alternative for certain keratoconus patients. The procedure can improve both uncorrected (UCVA) and best spectacle-corrected visual acuity (BSCVA), and it can reduce irregular astigmatism in corneas with and without scarring.25 A long-term study that followed the results of Intacs for keratoconus for five years found that the majority of patients experienced improved UCVA, BSCVA and refraction without evidence of progressive sight-threatening complications.26 Although the results are variable, some keratoconus patients after Intacs are able to avert corneal transplantation and use glasses or soft contact lenses to achieve adequate vision. Keratoconus patients who are undergoing Intacs should still expect to need rigid lenses afterwards to obtain the best vision.
More recently, some surgeons have used femtosecond lasers for creating the lamellar channels for inserting the Intacs segments, in lieu of using the mechanical spreaders.27-29 The femtosecond laser may improve the safety of Intacs and increase the acceptance of Intacs as a treatment for keratoconus.
New Contact Lens Treatment
Rigid gas-permeable (RGP) contact lenses are the primary therapy for restoring functional vision in keratoconus. The rigid lens surface creates a smooth, artificial surface to mask the underlying corneal irregularity, so that light can properly focus into the eye. Despite their optical function, RGP lenses do not retard the progression of keratoconus. In fact, several studies have implied that these lenses may even bring about keratoconus.30-32 Yet, because RGP lenses are the treatment for keratoconus and because early keratoconus can be subtle to detect, a causative relationship between RGP wear and keratoconus—if it even exists—may be impossible to prove or disprove.33 Keratoconus patients need not abandon RGP lens wear out of fear that it will exacerbate progression. The benefits from functional vision greatly outweigh any unproven concern about progression caused by lens wear.
There are several excellent proprietary RGP lens designs available for keratoconus, including Soper (David Thomas), McGuire (David Thomas), Rose K and Rose K2 lenses (Blanchard), IKone (Medlens and Valley Contax), NiCone (Lancaster), DynaZ+ nipple cone (Lens Dynamics), just to name a few.34 Larger overall diameter versions include Dyna Intra-Limbal (Lens Dynamics), GBL (ABB-Concise) and Rose K2 IC (Blanchard).
Because asymmetric inferior steepening of the cornea is commonly observed with keratoconus, inferior edge standoff can result in additional increased lens awareness. It is possible to prescribe Rose K and Rose K2 lenses using Blanchard’s Asymmetric Corneal Technology (ACT), where one (usually the inferior) quadrant of the lens is made with a steeper peripheral curvature to decrease edge lift in a specific site. There are other lens designs with similar technology as well—e.g., Dyna Intra-Limbal Quadrant Specific Technology (Lens Dynamics).
A relatively new fitting system is represented in the Clearion dual-hinge lenses (Acuity One), where there is an inner hinge point between the central base curve (BC1) and paracentral base curve (BC2) and an outer hinge point between BC2 and the peripheral curve. One Clearion lens design is the EP (ectasia profile), in which BC1 is significantly steeper than BC2. BC1 and BC2 can be specified independently to minimize apical bearing and achieve mid-peripheral alignment.
A common clinical challenge in managing keratoconus arises when the patient is unable to comfortably wear optimally prescribed RGP lenses. Piggybacking the best mechanically fit RGP lenses over soft lenses—where the posterior soft contact lens protects the underlying and sensitive corneal surface—has been one solution, especially in RGP lens-intolerant keratoconus patients, for 50 years.35 The recent availability of higher-Dk silicone hydrogel lenses frees the clinician from hypoxia concerns when prescribing such lens systems and allows for greater rates of clinical success.36
Another noteworthy new lens design that may help such patients is the SynergEyes ClearKone (SynergEyes). This hybrid lens was introduced in 2009 to accommodate a larger number of keratoconus patients, including those with decentered, oval and globus cones. ClearKone creates a significant tear reservoir to allow the rigid optics to vault over the distorted cornea, minimizing mechanical epithelial trauma during lens wear.
In situations where even the optimally prescribed hybrid lens causes unavoidable epithelial touch with related discomfort, one strategy is to piggyback the hybrid lens on a silicone hydrogel lens.37 Dr. Chou has successfully piggybacked more than two dozen keratoconus patients wearing the SynergEyes lenses with a silicone hydrogel carrier in situations where the best hybrid lens alone caused epithelial microtrauma and related discomfort.
Scleral contact lenses are not really new, nor are they novel in the management of keratoconus. In fact, one of the original uses of the early glass scleral lenses 100 years ago was in the optical treatment of keratoconus. RGP scleral contact lenses were developed by Don Ezikiel, O.D., and Perry Rosenthal, M.D., nearly a decade ago, and they have since become a viable—albeit often expensive and technically challenging—alternative to piggyback and hybrid lens designs.38,39 High-Dk RGP materials have allowed this advance.
The clinical goal of fitting an RGP scleral lens is to achieve complete apical corneal vault and modest clearance over the limbal region of the eye. Most clinicians start with a contact lens back radius of at least 1.00D steeper than corneal steep K and refine as needed until the clinician can see limbus-to-limbus clearance between the back of the lens and the anterior cornea in optic section with white light; fluorescein dye in the entrapped tears will assist this observation. The secondary goal is to rest the lens on the conjunctiva several millimeters beyond the limbus, so that there is neither too much physical pressure (as defined by blanching of the conjunctival vasculature over much of the circumference of the lens) nor too little, which would allow the entry of bubbles into the optical zone. Large immobile bubbles may lead to a decrease in vision, corneal desiccation and abrasion. Most of these lenses are manufactured in overall diameters of 15mm to 19mm, although some clinicians prefer even larger lenses, up to 24mm or more; lenses with overall diameters that are less than 15mm are usually avoided, as they tend to rest on the limbus.
Recent advances in instrument technology, as exhibited by such diagnostic tools like the Visante OCT (Carl Zeiss Meditec), provide a means of measuring corneal and scleral contour beyond the range of placido-based corneal topography. Bill Meyers, Ph.D., and Jerry Legerton, O.D., M.S., M.B.A., used the distribution of scleral contour data to design a novel mini-scleral lens (15.5mm), which holds promise of an improved scleral contact relationship along with reduced practitioner chair time and reordering.40 (See “It’s Time to Rethink Mini-Scleral Lenses” in this month’s Review of Optometry for more on this new lens design.)
Once a “reasonable” mechanical fit is achieved, over-refraction defines the optics for optimal vision just as in other paradigms of RGP lens practice, and evaluation during the adaptation phase will detect any needed alterations in optics or fit to achieve best results.
Keratoconus, Unnaturally
Keratoconus is the prototypical, naturally occurring eye condition characterized by corneal irregularity. Post-LASIK ectasia, while iatrogenic, is similarly managed with RGP lens optics. Certain patients with post-LASIK ectasia may benefit from Intacs surgery and/or CXL in the same way that certain keratoconus patients do.41,42 Although principles of contact lens and surgical management for keratoconus and post-LASIK ectasia are similar, in our experience, there are more emotional issues in post-LASIK ectasia patients, including anger, resentment and distrust. These patients may believe they have brought poor vision upon themselves by choosing elective surgery, or they may think they were misled into having unwise surgery. When managing patients complaining of poor vision after refractive surgery, eye care practitioners who provide rehabilitative contact lens treatment should take particular care in exam documentation. Unlike patients with keratoconus who typically accept their condition without blame, we have observed several of our post-LASIK ectasia patients initiate litigation against their refractive surgeon. In such instances, your exam records will be subpoenaed, and you will be called to testify.
The Antithesis of Commodity
The pattern of corneal irregularity—whether in keratoconus or other thinning disorders, corneal surgery, infection or trauma—is unique to each eye. That is why there can be no single lens design that accommodates every irregular cornea.
By comparison, disposable hydrogel and silicone hydrogel contact lenses follow a one-size-fits-all model, and the value of a practitioner’s clinical expertise is minimized by direct-to-consumer advertising in such cases. Coupons for free trial lenses strengthen the patient/product relationship and the do-it-yourself mentality among patients—while disrupting the relationship between patient and practitioner.
The beauty of prescribing contact lenses for irregular corneas is that clinical success stems from the practitioner’s knowledge and skill, rather than a result of a specific lens brand marketing campaign. While “specialty” contact lenses once included toric and multifocal contact lenses, this distinction is eroding. Fortunately, contact lens prescribing for irregular corneas is a bastion in our profession, where our services undeniably hold intrinsic value.
|
Quick Clinical Tips • Aggressively prescribe topical combination antihistamine/mast-cell stabilizers. Eye rubbing is common among keratoconus patients and is implicated in its genesis. The safest option is for keratoconus patients to refrain from rubbing their eyes. Sometimes it is easier said than done, which is why allergy eye drops for keratoconus patients are appropriate. • Glasses are not contraindicated. A surprising number of keratoconus patients benefit from glasses. Even if the vision with glasses is poor vs. that with RGP lenses, spectacles can help them function around the house if their eyes are too irritated to wear lenses. Additionally, keratoconic patients who already wear lenses may benefit from wearing glasses over their lenses to compensate for residual astigmatism. • Assume that keratoconus is bilateral, especially because some of these patients inquire about having LASIK in their “good” eye. Even if the disease appears to be unilateral, the likelihood is that the asymmetry is so great that your clinical measurements don’t have the sensitivity to detect its existence in the “good” eye. • Advise patients to let their relatives know that if they undergo a LASIK evaluation, they should mention that keratoconus exists in the family. • Direct keratoconus patients to the National Keratoconus Foundation website ( www.nkcf.org), which provides patients with information and support. • Edge design is critical for comfort and avoiding unwanted giant papillary conjunctivitis (GPC), which will minimize wearing time. Use special cleaners and avoid using steep, abrupt lenticulars with high-minus prescriptions. |
Dr. Chou is an industry consultant and private practitioner in San Diego with a clinical emphasis on contact lens prescribing for irregular corneas. Dr. Weissman is a professor of ophthalmology at Jules Stein Eye Institute, David Geffen School of Medicine at UCLA.
1. Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthal. 1986 Mar 15;101(3):267-73.
2. A barometer for recession. Available at: www.nytimes.com/2008/04/24/business/worldbusiness/24ihtlasik.1.12301419.html. (Accessed January 2010).
3. Ambrósio R, Klyce S, Wilson S. Corneal topographic and pachymetric screening of keratorefractive patients. J Refract Surg. 2003 Jan-Feb;19(1):24-9.
4. Barmaby FJ. Drop in Lasik eye surgery appears to be 4. Lema I, Durán JA, Ruiz C, et al. Inflammatory response to contact lenses in patients with keratoconus compared with myopic subjects. Cornea. 2008 Aug; 27(7):758-63.
5. Lema I, Durán JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005 Apr:112(4):654-9.
6. Lema I, Sobrino T, Durán JA, et al. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol. 2009 Jun:93(6):820-4.
7. Shah SS, Yeung KK, Weissman BA. Contact lens related deep stromal vascularization. Int Cont Lens Clin 1998 Sept;25(5):128-36.
8. Jafri B, Li X, Yang H, et al. Higher order wavefront aberrations and topography in early and suspected keratoconus. J Refract Surg. 2007 Oct:23(8):774-81.
9. Alió JL, Shabayek MH. Corneal higher order aberrations: a method to grade keratoconus. J Refract Surg. 2006 Jun:22(6):539-45.
10. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003 May;135(5):620-7.
11. Coskunseven E, Jankov MR, Hafezi F. Contralateral eye study of corneal collagen cross-linking with riboflavin and UVA irradiation in patients with keratoconus. J Refract Surg. 2009 Apr:25(4):371-6.
12. Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol. 2006 Aug:17(4):356-360.
13. Gokhale NS, Vemuganti GK. Diclofenac-induced acute corneal melt after collagen crosslinking for keratoconus. Cornea. 2010 Jan 29(1):117-9.
14. Raiskup F, Hoyer A, Spoerl E. Permanent corneal haze after riboflavin-UVA-induced cross-linking in keratoconus. J Refract Surg. 2009 Sep:25(9):S824-8.
15. Koppen C, Vryghem JC, Gobin L, et al. Keratitis and corneal scarring after UVA/riboflavin cross-linking for keratoconus. J Refract Surg. 2009 Sep;25(9):S819-23.
16. Boyle E. FDA backs launch of collagen cross-linking clinical trials. Available at: www.osnsupersite.com/view.aspx?rid=25785. (Accessed January 2010).
17. FDA trial data positive for corneal collagen crosslinking. Available at: www.modernmedicine.com/modernmedicine/Clinical+News/ASCRS-FDA-trial-data-positive-for-corneal-collagen/ArticleStandard/Article/detail/591528?contextCategoryId=46496&ref=25. (Accessed January 2010).
18. Tuft SJ, Moodaley LC, Gregory WM. Prognostic factors for the progression of keratoconus. Ophthalmology. 1994 Mar;101(3):439-47.
19. Brierly SC, Izquierdo L, Mannis MJ. Penetrating keratoplasty for keratoconus. Cornea. May 2000:19(3):329-332.
20. Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002 Mar;28(3):398-403.
21. Han DC, Mehta JS, Por YM, et al. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009 Nov;148(5):744-751.
22. Bahar I, Kaiserman I, Srinivasan S, et al. Comparison of three different techniques of corneal transplantation for keratoconus. Am J Ophthalmol. 2008 Dec;146(6):905-12.
23. Watson SL, Ramsay A, Dart JK, et al. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology. 2004 Sep:111(9):1676-82.
24. Colin J. Intacs may be useful for select keratoconus correction. Ocular Surgery News April 15, 1999.
25. Boxer Wachler BS, Christie JP, Chandra NS, et al. Intacs for keratoconus. Ophthalmology. 2003 May;110(5):1031-40.
26. Zkymionis GD, Siganos CS, Tsiklis NS, et al. Long-term follow-up of Intacs in keratoconus. Am J Ophthalmol. 2007 Feb:143(2):236-244.
27. Rabinowitz YS. Intacs for keratoconus. Curr Opin Ophthalmol. 2007 Jul;18(4):279-83.
28. Rabinowitz YS, Li X, Ignacio TS, et al. Intacs inserts using the femtosecond laser compared to the mechanical spreader in the treatment of keratoconus. J Refract Surg. 2006 Oct:22(8):764-71.
29. Ertan A, Kamburoglu G, Bahadir M. Intacs insertion with the femtosecond laser for the Management of keratoconus: one-year results. J Cataract Refract Surg. 2006 Dec:32(12):2039-42.
30. Hartstein J. Keratoconus that developed in patients wearing corneal contact lenses: report of four cases. Arch Ophthalmol. 1968 Sep;80(3):345-6.
31. Brady HR. Keratoconus development in a contact lens wearer. Cont Lens Med Bull 1972;5:23.
32. Brightbill FS, Stainer GA. Previous hard contact lens wear in keratoconus. Cont Int Lens Med J. 1979;5(3):4347.
33. Zadnik K, Barr JT. Diagnosis, contact lens prescribing, and care of the keratoconus patient. Boston. Butterworth-Heineman; 1999:30.
34. Caroline PG, McGuire JR, Doughman DJ. Preliminary report on a new contact lens design for keratoocnus. Contact Intraoc Lens Med J 1978;4:69-73.
35. Westerhout D. The combination lens and therapeutic uses of soft lenses. Contact Lens J. 1973;4:3-10.
36. Weissman BA, Ye P. Calculated tear oxygen tension under contact lenses offering resistance in series: piggyback and scleral lenses. Cont Lens Anterior Eye. 2006 Dec;29(5):231-7
37. Scheid T, Kaplan E. A novel keratoconic piggyback fitting utilizing a SiH lens and a Synergeyes KC Hybrid. Available at: www.siliconehydrogels.org/in_the_practice/mar_08.asp. (Accessed January 2010).
38. Schein OD, Rosenthal P, Ducharme C. A gas permeable scleral contact lens for visual rehabilitation. Am J Ophthalmol. 1990 Mar 15;109(3):318-22.
39. Schornack MM, Patel SV. Scleral lenses in the management of keratoconus. Eye Contact Lens. 2010 Jan;36(1):39-44.
40. Personal communication with William E. Meyers, Ph.D., on March 12, 2010
41. Pinero DP, Alio JL, Eceda-Montanes A, et al. Intracorneal ring segment implantation in corneas with post-laser in situ keratomileusis keratectasia. Ophthalmology. 2009 Sep;116(9):1665-74.
42. Winciquerra P, Camesasca F, Albé E, et al. Corneal collagen cross-linking for ectasia alter excimer laser refractive surgery: 1-year results. J Refract Surg. 2009. Sep 22:1-12.


