 |
 |
A 65-year-old man presented for a scheduled six-month post-op follow-up of Descemet’s stripping automated endothelial keratoplasty (DSAEK) OS. He was last seen three months prior, at which point the transplant was healthy and his vision was recovering nicely. Prednisolone acetate 1% had been tapered from QID to TID and he was asked to return in another three months. At that time, he reported good vision and comfort and had not noted any redness or irritation.
Vision was down from the 20/25 via autorefraction measured at his previous visit to 20/40 with no improvement on pinhole. IOP was 19mm Hg, and slit lamp OS showed normal lids and a white eye. His cornea had trace diffuse stromal edema and a well-centered DSAEK graft with 3+ diffuse keratic precipitates (KPs) scattered on the graft endothelium. The anterior chamber had trace cell, and deeper structures were unremarkable.
A diagnosis of corneal allograft rejection following DSAEK was made and we increased prednisolone to hourly. He was not followed up as closely as we prefer because he didn’t live nearby—he traveled three hours to be seen—and instead was asked back in seven days. However, he was instructed to call sooner if he noticed deterioration of vision, worsening of comfort or increasing redness.
At follow-up, vision was 20/30, IOP was stable (18mm Hg) and the rejection episode was resolving, with reducing KP. He was asked to taper his steroid to Q2h for two weeks and return for evaluation. At this follow-up, the episode appeared to have resolved, with 20/20 vision on autorefraction and full resolution of KP and edema. The patient was asked to reduce his steroid to QID; however, he was instructed that we would not be reducing steroids further for up to a year.
 |
|
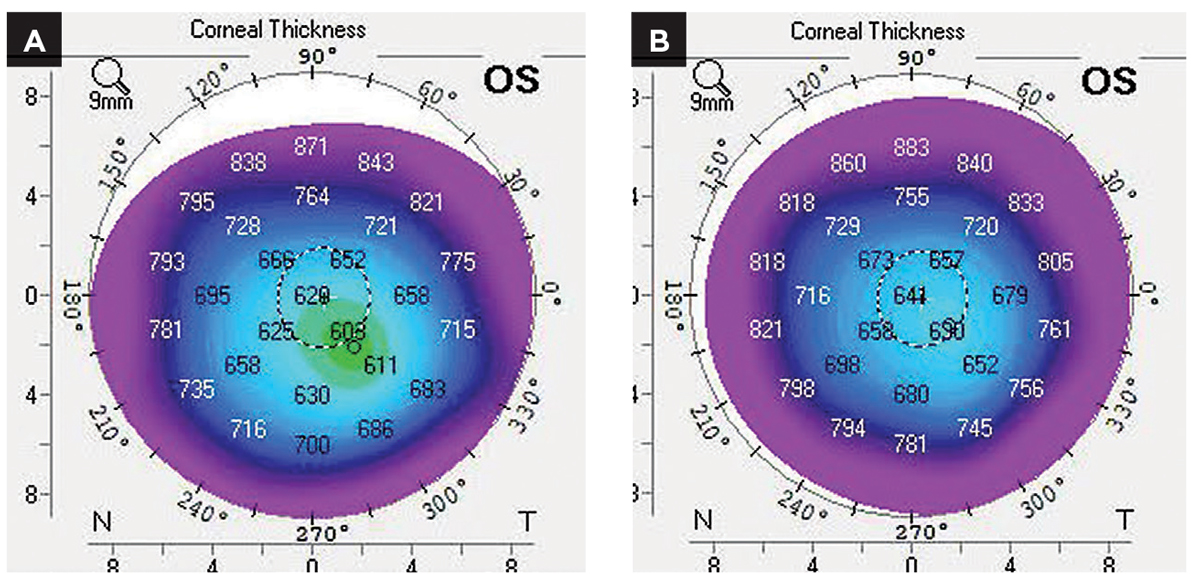
(A) Pachymetric map at rejection. (B) Pachymetric map at resolution. Comparison of the two shows resolution of mild edema. Click image to enlarge. |
Reasons for Rejection
Rejection of an allograft is possible in any donor-derived transplanted tissue containing cells; it indicates the host immune system has become sensitized to the donor tissue. This leads to a CD-4+ driven immune infiltration to the graft, where the donor nucleated cells are targeted. Though corneal transplants are at lower risk for rejection compared with most tissue or organ transplants owing to both passively and actively regulated immune privilege, rejection can occur.1
In intense episodes or those treated late in the process, rejection can precipitate failure of the graft. In most cases, rejection-induced failure results from direct attack and collapse of the donor endothelium, though resultant vascularization and scarring can also occasionally cause failure. Of all types of corneal transplants, penetrating keratoplasties (PKs) are most likely to both reject (20% incidence) and fail as a result of rejection, as rejection is the leading cause of premature graft failure of a PK.2 Though a deep anterior lamellar keratoplasty (DALK) transplant looks identical to a PK, the lack of donor endothelium makes rejection-based failure exceedingly rare.3
Finally, the risk of rejection and failure of the endothelial transplants DSAEK and Descemet’s membrane endothelial keratoplasty (DMEK) falls somewhere between the two extremes of PK and DALK.4,5 Despite the presence of donor endothelium in the DSAEK and DMEK, these grafts both have lower rates of rejection, as well as lower rates of rejection-induced failure compared with PK as they erode immune privilege of the cornea less than PK. Of the two, DSAEK is more likely to reject.5 In cases of rejection of donor endothelium, the clinical appearances are KPs—generally in a diffuse pattern—but occasionally may take on a linear conformation and/or corneal edema overlying the zones of KP.
 |
|
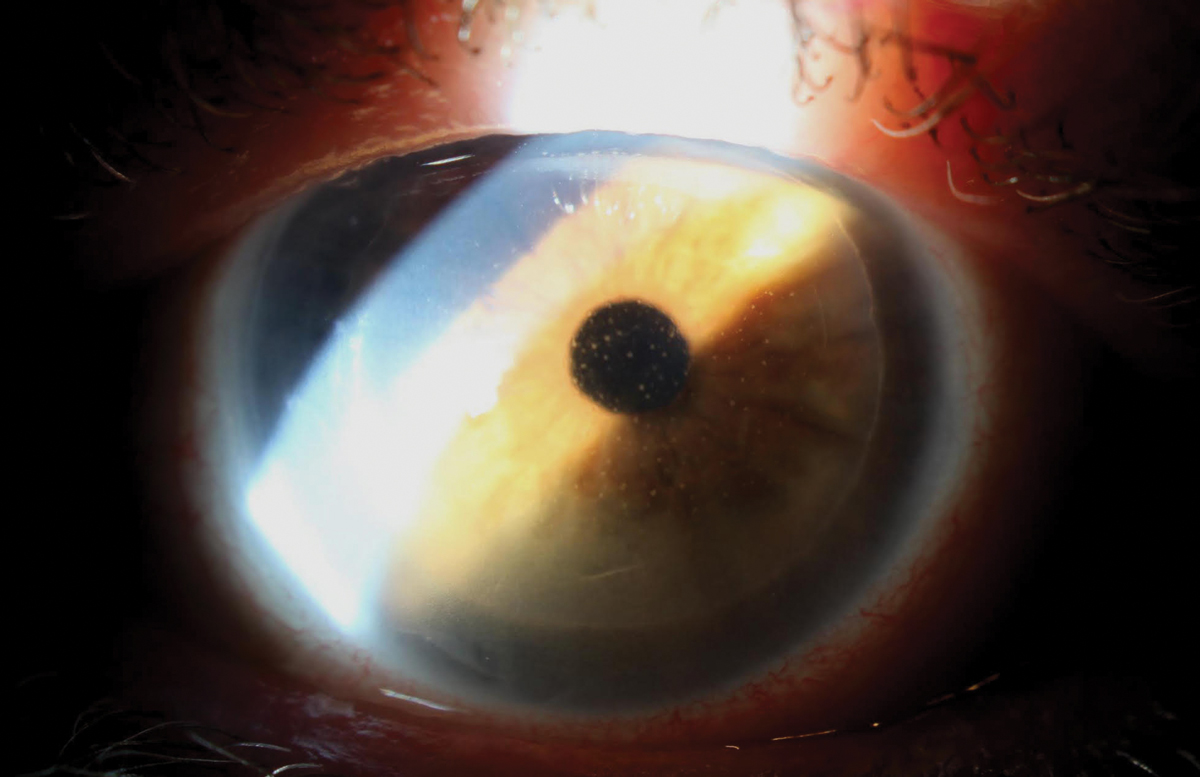
Corneal rejection of DSAEK graft. KP is the diagnostic sign here.Click image to enlarge. |
An Ounce of Prevention
The primary treatment of corneal graft rejection is prevention. Following a corneal transplant, patients are placed on a corticosteroid in order to suppress the local immune presence. Studies have found that corneal allograft rejection is most likely to occur in the first several months following transplant, with reduced odds the further one moves beyond the early time period.
This trend of rejection over time has to do with an interesting process called immune tolerance, where the body’s immune system is aware of the foreign tissue—but doesn’t attack it. Immune tolerance of foreign antigen is possible across various locations of the body but is among the most naturally achievable with corneal grafts, which benefit from the pro-tolerance/immune-privileged status of the cornea.6,7 Immune privilege status of the cornea can most easily be appreciated by recognizing that corneal transplants had been successfully performed many decades prior to the advent of immune modulation with topical glucocorticoids.
Immune privilege is derived from several different features of the tissue, but of particular interest is one called anterior chamber–associated immune deviation (ACAID). Though the full mechanism of ACAID is well beyond the scope of this column, it is a fascinating feedback cycle, whereby foreign antigens discovered through the anterior chamber and result in a population of T-cells that not only do not attack the antigen/graft, but also actively suppress immune response against it.7,8 As previously stated, rejection of a corneal transplant is most likely to occur over the first few months postoperatively. This can be partially explained by a delay in the development of the ACAID, which takes time to establish.
Following uneventful surgery and early recovery with posterior lamellar transplants, topical steroid is tapered over a period of 12 to 24 months, depending on surgery center preference. In cases of graft rejection, where both immune tolerance and prevention dosing with steroid fail, increased immune suppression is needed. This is typically achieved with increased dosing of topical corticosteroids, though an oral corticosteroid or compounded topical cyclosporin A or tacrolimus may also be used.
Dosing should be initially high—every hour or more—with a gradual taper.9,10 The final level of that taper will vary from case to case. I (Dr. Bronner) always use the benchmark of steroid dose at the time of rejection and plan on staying above that level for at least a year, depending on other variables, including the patient’s response to steroids. Ocular surface disease, herpetic infection and lid margin health all play a role as well.
In this case, the patient’s modest endothelial rejection of his DSAEK graft was able to be treated successfully with an increased corticosteroid. Following taper of the original rejection treatment, he was maintained on elevated dosing (relative to what he was on prior to rejection) for 12 months prior to reducing the medication further with three-month follow-up intervals over that time period. Today, two years after the rejection episode, the patient continues to be gradually weaned off the steroid and is maintaining at QD dosing.
1. Niederkorn JY. Mechanisms of corneal graft rejection: the sixth annual Thygeson Lecture presented at the Ocular Microbiology and Immunology Group meeting, October 21, 2000. Cornea. 2001;20(7):675-9. 2. Sugar A, Tanner JP, Dontchev M, et al. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009;116(6):1023-8. 3. Cheng YY, Visser N, Schouten JS, et al. Endothelial cell loss and visual outcome of deep anterior lamellar keratoplasty vs penetrating keratoplasty: a randomized multicenter clinical trial. Ophthalmology 2011;118(2):302-9. 4. Patel SV. Graft survival and endothelial outcomes in the new era of endothelial keratoplasty. Exp Eye Res. 2012;95(1):40-7. 5. Anshu A, Price MO, Price F. Risk of corneal transplant rejection significantly reduced with DMEK. Ophthalmology 2012;119(3):536-40. 6. Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopatheogenesis of microbial keratitis, peripheral ulcerative keratitis and corneal transplant rejection. Cornea. 2000;19(5):625-43. 7. Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3(11):879-89. 8. Niederkorn JY, Mellon J. Anterior chamber-assoicated immune deviation promotes corneal allograft survival. Invest Ophthalmol Vis Sci. 1996;37(13):2700-7. 9. Sinha R, Jhanji V, Verma K, et al. Efficacy of topical cyclosporine A 2% in prevention of graft rejection in high-risk Keratoplasty: a randonmized controlled trial. Graefes Arch Clin Exp Ophthalmol. 2010;248(8):1167-72. 10. Javadi MA, Feizi S, Karbasian A, Rastegarpour A. Efficacy of topical ciclosporin A for treatment and prevention of graft rejection in corneal grafts with previous rejection episodes. Br J Ophthalmol. 2010;94(11):1464-7. |


