 |
 |
A 68-year-old woman presented to the emergency department with a history of intermittent redness and pain in the left eye for one month.
She had a past ocular history of radial keratotomy (RK) in both eyes and a penetrating keratoplasty (PKP) eight months prior in the left eye, for which she was using prednisolone acetate 1% twice daily.
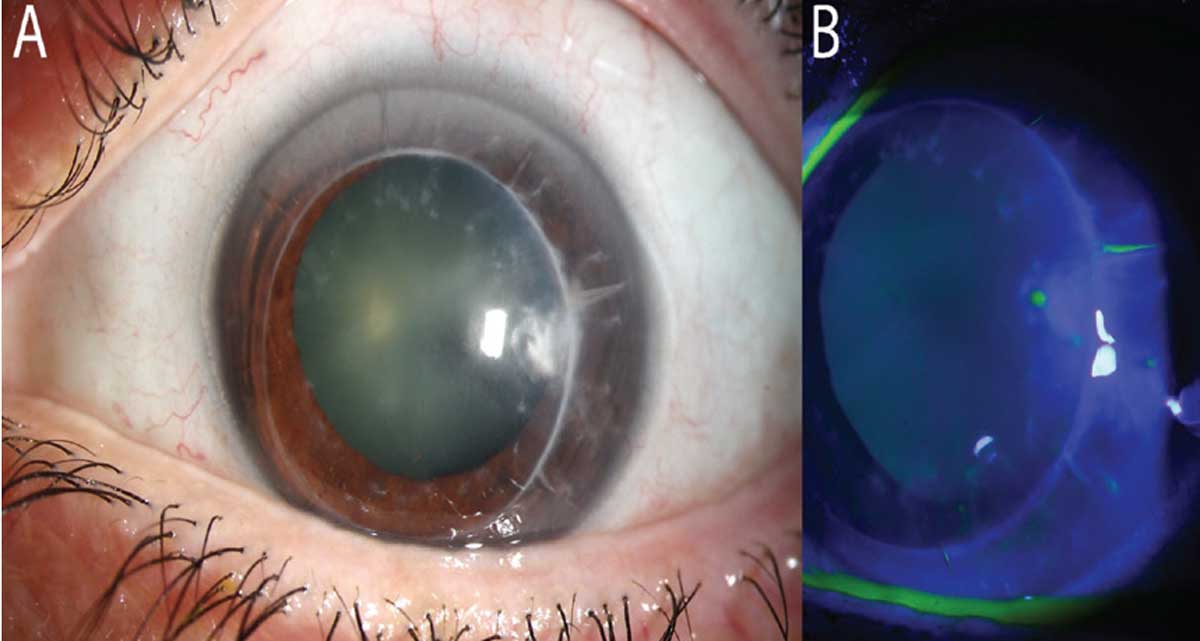
On examination, her vision pinholed to 20/80 in the right eye and 20/60 in the left eye. Intraocular pressure and dilated fundus examination were within normal limits in both eyes. The anterior segment exam of the right eye revealed RK scars and moderate endothelial guttae without stromal edema, and the left eye revealed RK scars on the host cornea and a PKP with no sutures remaining. The graft was grossly clear, but attention was drawn to the temporal graft, where a fluffy deep stromal infiltrate was appreciable below a prior suture site. There was an overlying pinpoint epithelial defect. The left eye’s anterior chamber revealed 1+ cell and flare, but the conjunctiva was surprisingly quiet (Figure 1). Further imaging with anterior segment optical coherence tomography (OCT) revealed a hyperreflective density at 50% depth (Figure 2).
Upon review of the patient’s medical records, there had been an eroded corneal suture temporally on the left eye one month prior. The suture was removed at that time, but the patient noted her symptoms had since worsened.
 |
|
Fig. 1. (a) Minimal conjunctival injection (post phenylephrine/dilation). Notice the PKP graft with host RK scars. At 3 o’clock, there is a deep but irregular infiltrate. (b) Notice the lack of NaFI staining over the lesion, denoting only a pinpoint epithelial defect where the graft’s suture passed. Click image to enlarge. |
Microenvironment
PKPs change the corneal dynamics. First, the corneal innervation is significantly altered. During surgery, full-thickness trephination of the diseased cornea severs corneal nerves. Corneal innervation is integral for a number of processes, including protection of the ocular surface via blink reflexes, enabling wound healing by numerous trophic mechanisms and providing neural feedback to stimulate lacrimation.1,2
Studies reveal that, following PKP, a peripheral graft’s subbasal nerve complex may begin to reform in as little as two months, but central innervation may take one to two years before it is initially detectable.3,4 Even when detectable, significant alterations in the density and branching pattern of the subbasal nerve complex exist, and there remains some degree of decreased sensation in many cases.4,5
Another alteration in the PKP microenvironment is local immunosuppression. Though the cornea may have relative immune privilege compared to other areas of the body due to lack of corneal neovascularization or lymphatics, corneal graft rejection still remains a real risk. It has been well-accepted that local or systemic immunosuppression greatly reduces the chance of graft rejection.6,7 In most cases this is successfully done with topical ophthalmic corticosteroids, but topical cyclosporine and tacrolimus, as well as oral immunosuppressives such as mycophenolate mofetil and cyclophosphamide, have been used successfully.8 Regardless of the drug, the result is a reduced host immune response against the donor tissue or exogenous infectious sources.
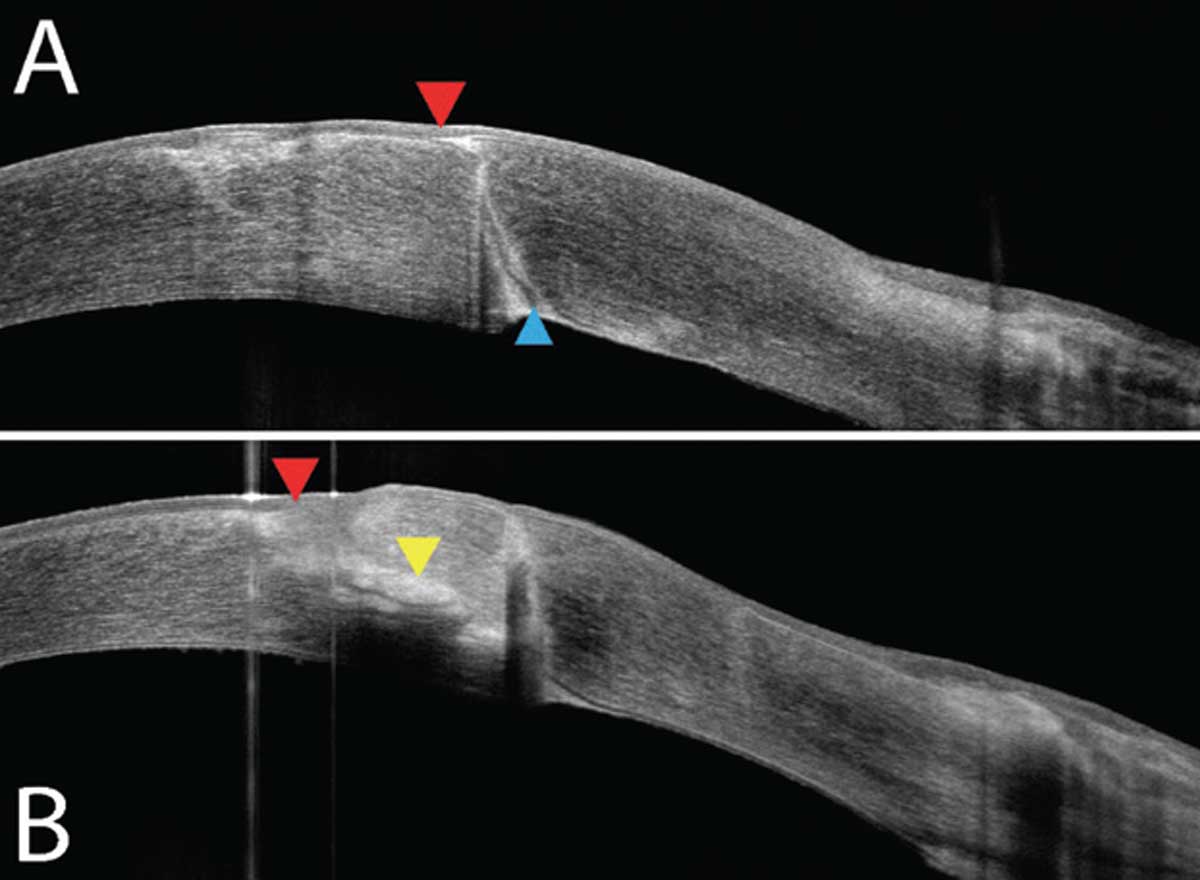 |
| Fig. 2. (a) OCT of the graft-host junction superior to the infiltrate shows a fully intact epithelium (red) and a delineated graft-host barrier (blue). (b) OCT over the lesion demonstrates intact corneal epithelium (red) with a midstromal opacity/infiltrate (yellow). Click image to enlarge. |
Finally, corneal sutures are always placed during full-thickness keratoplasties—typically first in four cardinal positions, then the graft is further stabilized by the use of a single running suture or multiple interrupted sutures. Sutures can remain in the graft indefinitely but are often removed for refractive optimization. While in place, they may break or loosen with time. Exposed sutures lead to a breakdown of the corneal epithelial barrier and can allow a pathway for external pathogens to make their way into the corneal stroma.
Corneal Sutures and Abscess Formation
In addition to assessing the overall status of the corneal transplant itself, clinicians should carefully evaluate any sutures. The best way to visualize a suture’s integrity is to use topical sodium fluorescein (NaFl). A suture properly covered by epithelium will not stain under blue light. A loose or eroded suture, on the other hand, will stain with NaFl. Though NaFI may pool over a suture mimicking epithelial erosion, it’s helpful to draw up any excessive dye from the suture with a sterile cotton-tipped applicator or cellulose sponge spear for better evaluation. If the NaFl can be soaked up and there is no staining of the suture, the suture is not exposed and may be left in place. Any that are loose, broken or otherwise exposed, should be removed. Most physicians recommend using povidone-iodine solution prior to suture removal, then prophylactic topical antibiotics are dosed until the epithelial defect is closed.
The presence of corneal sutures, in addition to reduced corneal innervation and chronic local immunosuppression, combine to create a corneal microclimate that predisposes the post-keratoplasty eye to microbial keratitis.9 The rate of infectious keratitis (IK) among eyes that have undergone a PKP is high, anywhere from 1.76% to 11.9%.10 Suture tract abscesses, in particular, occur when a suture becomes exposed to the external environment and allows for microbial penetration deeper into the stromal tissue. These appear clinically as corneal infiltrates along any portion of the suture tract, usually with attendant anterior chamber inflammation and conjunctival injection.
There will often be a small epithelial defect, but the absence of one does not exclude the presence of an infection. Patients will likely complain of photophobia, foreign body sensation and decreased vision. Loose or eroded sutures, suture manipulation and suture abscesses have all been implicated in the development of endophthalmitis, which generally carries a poor visual prognosis.11
Patient Outcome
Due to the presence of a deep stromal infiltrate, a cornea specialist was called for evaluation. In most IK cases, a corneal culture is taken by scraping an ocular surface lesion with a surgical spatula/blade or sterile cotton alginate swab. Because of the depth of infection, a culture by suture pass was performed. The surgeon anesthetized the eye, then passed a curved needle with braided silk suture in through clear cornea adjacent to the infiltrate. Next, the needle and suture were directed anteriorly through the infiltrate. The suture material was plated on blood agar, chocolate agar, Sabouraud dextrose agar, Lowenstein-Jensen medium and agar agar, and sent to the microbiology laboratory.
Two days later, the culture grew a yeast, Candida parapsilosis. Fortified antibiotics originally dosed every hour were reduced to four times daily and natamycin 5% was started every hour. At one week, the stromal infiltrate was thought to be worsening, so a superficial scrape was performed to allow for improved drug penetration. Given the potential of Candida parapsilosis to form biofilms, fortified topical voriconazole and oral fluconazole were added. The patient was evaluated weekly for two months before the infection was finally ameliorated. Fortunately, this post-keratoplasty eye had a good visual outcome.
Managing patients with corneal transplants can certainly have its ups and downs. It’s important to be aware of the abnormal microclimate in these transplants and be vigilant in searching for signs of complications. Early recognition and removal of loose or broken sutures can prevent worse outcomes by reducing the risk of inflammation and infection.
1. Müller LJ, Marfurt CF, Kruse K, Tervo TMT. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):21-42. 2. Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. Ocul Surf. 2004;2(2):76-91. 3. Richter A, Slowik C, Somodi C, et al. Corneal reinnervation following penetrating keratoplasty-correlation of esthesiometry and confocal microscopy. Ger J Ophthalmol. 1996;5(6):513-7. 4. Darwish T, Brahma A, Efron N, O’Donnell C. Subbasal nerve regeneration after penetrating keratoplasty. Cornea. 2007;26(8):935-40. 5. Stachs O, Zhivov A, Kraak R, et al. Structural-functional correlations of corneal innervation after LASIK and penetrating keratoplasty. J Refract Surg. 2010;26(3):159-67. 6. Shimmura-Tomita M, Shimmura S, Satake Y, et al. Keratoplasty postoperative treatment update. Cornea. 2013;32 Suppl 1:S60-4. 7. Abudou M, Wu T, Evans JR, Chen X. Immunosuppressants for the prophylaxis of corneal graft rejection after penetrating keratoplasty. Cochrane Database Syst Rev. 2015;(8):CD007603. 8. Azevedo Magalhaes O, Shalaby Bardan A, Zarei-Ghanavati M, Liu C. Literature review and suggested protocol for prevention and treatment of corneal graft rejection. Eye (Lond). 2020;34(3):442-50. 9. Leahey AB, Avery RL, Gottsch JD, et al. Suture abscesses after penetrating keratoplasty. Cornea. 1993;12(6):489-92. 10. Vajpayee RB, Sharma N, Sinha R, et al. Infectious keratitis following keratoplasty. Surv Ophthalmol. 2007; 52(1):1-12. 11. Henry CR, Flynn HW Jr, Miller D, et al. Delayed-onset endophthalmitis associated with corneal suture infections. J Ophthalmic Inflamm Infect. 2013;3(1):51. |


