There are about 125 million contact lens wearers worldwide.1 Excluding the daily disposable and extended wear contact lenses, all other types of lens wear modalities require the use of a contact lens storage case for overnight storage and disinfection. However, the contact lens storage case itself can become contaminated by pathogenic micro-organisms.2
Contact lens storage case contamination is common in lens wearers, occurring in 30% to 85% of the cases.2-7 Studies have shown that lens cases receive the least cleaning attention and are the most frequently—and heavily—contaminated items compared to other lens care accessories.5,8-10
A variety of organisms have been identified in contact lens storage cases, including different types of bacteria, viruses, fungi and protozoans.2,3,11,12 These organisms include Pseudomonas, Serratia, Staphyloccus, Acanthamoeba and Fusarium species.3,5,11,13-15 Colonization of the lens storage case by pathogenic micro-organisms predisposes lens wearers to microbial or sterile keratitis.9,19-22
Poor adherence to the recommended cleaning regimen is believed to be the main cause of lens case contamination. Yet, studies have shown that even carrying out the recommended instructions does not necessarily guarantee a lens case free from contamination.21,23 Factors other than hygiene behaviors, including microbial factors such as biofilm formation and microbial resistance, may be associated with persistent microbial contamination of contact lens storage cases.24,25
Biofilm Formation in Lens Cases

Contact lens storage case.
Once bacteria enter the lens case, they may adhere to the case and switch from a planktonic (free-floating) phenotype to a sessile biofilm phenotype in response to a low-nutrient environment.24 The micro-organisms are embedded in a glycocalyx, which is a polysaccharide-containing matrix.24 Initially, this biofilm can be easily removed due to the loose attachment of cells.
But, the adhesion between microbial cells and the contact lens case surface becomes more persistent over time. The mature biofilm is significantly more resistant to antimicrobial agents than planktonic cells.26,27
Although contact lens multipurpose solutions or systems meet the international standard ISO 14729 and FDA 510(k) for adequate antimicrobial efficacy against selected reference strains of bacterial and fungal pathogens, antimicrobial efficacy varies among disinfecting solutions in vitro.6,28-31 In addition, the biocidal activity against biofilm and clinical isolates is unknown and both inherent and acquired microbial resistance may occur.23,25,32
In other words, simply soaking lens cases in disinfecting solutions may not be adequate to eliminate potentially virulent micro-organisms.7,24,32 Such factors represent a challenge in maintaining contamination-free storage cases; so, consider additional strategies to minimize storage case contamination.24,33
Today’s Approved Instructions
A recent study presented a systematic review of the Food and Drug Administration (FDA) and lens care manufacturers guidelines, and a survey of eye care practitioners’ lens case cleaning instructions for lens wearers.34 While the FDA advises lens cases be rinsed with disinfecting solution, air-dried face down after use, and replaced every three to six months, eye care practitioners and manufacturers provided less consistent advice.
For example, 20% of practitioners did not specify rinsing or air-drying lens cases. For those who recommended air-drying, 50% recommended drying cases face-up.34 There was also a large discrepancy between recommended timing of lens case replacement, ranging from one to six months between manufacturers and practitioners.
Although most of the two-flat well lens cases need to be air-dried after use, the silver-impregnated lens cases reportedly perform better when recapped, and this was not explicitly stated in the guidelines.35
The variation in advice from each source may increase wearer confusion. This perhaps explains that the major reported behaviors in lens wearers include not rinsing lens cases with disinfecting solution after use, not air-drying lens cases and not replacing lens cases regularly.5,8,36-40
In vitro studies reported that despite following the FDA and manufacturers’ recommended lens case cleaning instructions, these methods were actually not adequate in removing biofilm where a lens case is heavily contaminated.23,30 This finding confirms previous studies suggesting that even when a lens wearer follows the recommended guidelines, this may not result in contamination-free storage cases.21,23
Further, there have been controversies over whether the lens case should be cleaned with a cotton swab or toothbrush, boiled after use or microwaved; and, if so, how often.3,41-43 While these additional cleaning methods seem helpful in the attempt to reduce microbial contamination, concerns arise regarding the standardization of methods and the evidence supporting their use. In addition, their applicability across the myriad care systems and storage case types and designs, is unclear.
Although it is a responsibility of eye care professionals to deliver appropriate and consistent lens care recommendations to lens wearers, this may be challenging due to limited evidence-based lens case cleaning instructions. This suggests that we need further research to understand optimal storage case hygiene for lens wearers, and also how best to provide this information to eye care practitioners and to contact lens wearers.
Evidence-Based Guidelines: What Do We Know?
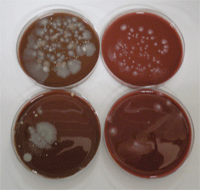
Micro-organisms recovered from a
contact lens storage case.
Microbial contamination often occurs when handling lenses, so hand-washing with soap is essential in minimizing contamination.44-46 The use of fresh disinfecting solution is recommended because topping-off the solution is associated with higher levels of contamination and lens-related complications.47,48 Simply soaking lens cases for the recommended time may not be sufficient to limit microbial contamination as discussed earlier.30 Rubbing lens cases followed by tissue-wiping is effective in removing bacterial bioburden from lens cases in vitro.30
Based on the available in vitro data, it seems reasonable that, after rinsing lens cases with fresh disinfecting solution, lens cases should be rubbed with clean fingers and further wiped with a clean tissue because such mechanical friction has been shown to reduce biofilm in lens cases.30
After the lens case has been cleaned, it needs to be air-dried face down. An in vitro study comparing the effectiveness of recapping vs. air-drying of conventional polypropylene lens cases demonstrated that recapping the lens cases resulted in significantly higher levels of bacterial recovery than air-drying cases.30 These data are consistent with epidemiological data showing that failure to air-dry lens cases is a risk factor for developing microbial keratitis.49
Lens cases air-dried face up in the bathroom were more frequently contaminated than those dried in a bedroom/office setting.50 However, air-drying location was less relevant if the cases are air-dried face down as this not only minimizes the exposure to air-borne contaminants but also promotes faster drying of lens wells, as suggested by the FDA.51
However, silver-impregnated lens cases perform better when recapped since maintaining some moisture in the lens case allows for the exchange of silver ions.35 So, recapping lids after use is recommended for silver-impregnated lens cases.
Lens storage case age is a proven risk factor for contact lens related corneal infection and older storage cases show higher levels of microbial contamination.7,49,52 Lens cases may develop moderate or heavy contamination, particularly by Gram-negative bacteria, even just after two weeks of use.52 This suggests that frequent replacement (at least monthly) of lens cases is recommended.7,52
The Challenge of Improving Lens Case Hygiene
The degree to which lens wearers’ hygiene practices coincide with practitioner advice has been defined as compliance. This rather narrow definition somewhat limits the autonomy of the lens wearer and excludes them from participating in decisions relating to their lens wear. Compliance is not a static concept; behaviors relate to perception of risks, health beliefs and values, wearer personality and the quality of the interactions between the practitioner and the wearer.
Lens wear is an ongoing activity and behaviors may change over time. Practitioners need to be aware of the factors that influence hygiene behaviour and the relevance of the mutual agreement or concordance between the wearer and the practitioner in the responsibility for outcomes in lens wear. If the wearer is excluded from the process, improving and indeed measuring compliance is difficult.
“Patient compliance” was the term originally used to describe the behavior of following recommendations prescribed by their clinicians. This implies a clinician-centered approach, and places patients in a passive role. By contrast, adherence connotes a patient’s commitment to a regimen in an active, voluntary and collaborative way. Past efforts by practitioners have generally focused on improving practice through education and information. Despite these efforts, the level of hygiene practice remains low.8,36,37,53,54 Improving contact lens case hygiene has been a difficult challenge for eye care practitioners.
The underlying causes of non-adherence to recommendations are probably diverse and complex. It may be that lens wearers follow outdated instructions, are not receiving adequate instructions from eye care practitioners in the first place, are confused by different recommendations from various sources or choose not to follow instructions due to their perception of the impact of the risk of non-adherence to recommended hygiene regimen.34,36

Addressing Non-Adherence
While this is a complex issue, it may be helpful to consider some potential issues and strategies for wearer communication and engagement.
• Lens wearers not aware of the updated or adequate instructions.
The majority of non-adherence in lens wearers appears to be due to a lack of awareness of adequate cleaning procedures or the importance of storage case cleaning.36,54 During a contact lens consultation, a practitioner is required to assess the fitting, comfort and performance of contact lenses. At the same time, the lens wearer is required to process and retain a large amount of information—e.g. lens replacement schedule, how to insert/removal lenses, how to clean, what solution to use, and so on. The volume of information for the wearer to retain can be overwhelming. On average, patients retain just 50% of verbal instructions after a consultation, and 40% to 80% of instructions are forgotten immediately under certain circumstances.55,56
The understanding of proper use of the lens products in lens wearers needs to be confirmed by the practitioners—either by asking open questions or behavioral observations such as asking the lens wearers to demonstrate how they carry out their lens care. The use of visual tools, such as demonstrating cleaning techniques for lens wearers in the clinic, and take-home instructions in addition to verbal communication, can enhance knowledge retainment.56,57
Follow-up care provides an opportunity to revisit lens wearers’ hygiene habits and their use of contact lenses, care products and, in particular, the maintenance of their lens case. An open-ended question approach helps to elicit lens wearers’ hygiene practices, understanding and concerns of lens wear; this allows for early detection and modification of any inappropriate lens care. Unfortunately, more than half of lens wearers fail to recognize the importance of follow up care.37,53 Regular follow up should be addressed by the practitioners regardless of whether or not the contact lens wearers are experienced in lens handling and satisfied with the performance of their contact lenses.
• Lens wearers can be confused with different recommendations from various sources.
Non-adherence to recommended hygiene regimens in lens wearers ranges from 50% to 79%.54 Almost one third of this non-adherence is associated with lens case hygiene, which suggests a lack of consistent education and absence of standarized guidelines as mentioned previously.34,36
We strongly urge eye care practitioners, academia and industry to collaborate to develop consistent and evidence-based cleaning guidelines. Such guidelines need to be effective in removing microbial biofilms and only require minimum additional paraphernalia, which is important for improving hygiene commitment in contact lens wearers. Manufacturers can also support the hygiene strategies by emphasizing the lens and lens case cleaning instructions on the solution bottle where they are easily accessed and highly visible.
Lens wearers commonly switch care systems due to convenience or cost issues without consulting with their eye care practitioners. In doing so, lens wearers may not realize that case cleaning procedures sometimes differ between products. So, it is essential to explain the rationale for prescribing a specific system and to specify the prescribed care system on the contact lens prescription.
• Intentional non-adherence.
It is possible that lens wearers do not perceive lens-related complications as sight threatening, and they often do not experience immediate harm from their non-compliant behavior. So, lens wearers don’t have sufficient incentive and motivation to change their behaviour.53 Inevitably, some lens wearers are always more resistant to standard care and recommendations about appropriate contact lens hygiene despite all efforts.
A large proportion of lens wearers do not follow a recommended hygiene regimen despite awareness of risk.58 One study found that intrinsic characteristics, such as the risk-taking propensity of individuals, may influence the care and maintenance of contact lenses and, even though those individuals are aware of health hazards, they still tend to perform the risky behavior.59,60
Contact lens wearers make up a different demographic compared to our other patient groups and may require alternative education approaches.37,53,61 Perhaps in the future, a tool such as a personality assessment may be useful to practitioners to help with the lens wearer selection process and the development of more targeted cleaning regimen enhancement strategies.
In summary, contact lens storage cases can become contaminated by microorganisms, resulting in microbially-driven adverse events such as corneal inflammation and infection. Current recommendations for lens case cleaning are not effective in preventing or limiting contamination and particularly in removing microbial biofilms. Limiting lens storage case contamination may reduce adverse events and result in safer contact lens wear but solving this complex problem requires collaboration between eye care practitioners, academia and industry in the development of evidence-based cleaning guidelines. Additionally, further development of lens care products, perhaps those targeting specific microbial virulence factors may assist, plus a commitment is required from advisory sources to disseminate comprehensive and consistent lens case cleaning guidelines. Finally, adherence to lens and storage case cleaning instructions depends on collaboration and understanding between contact lens practitioners and contact lens wearers.
Professor Fiona Stapleton is the Head of the School of Optometry and Vision Science at the University of New South Wales and Senior Research Associate at the Brien Holden Vision Institute at the University of New South Wales, Sydney, Australia.
Yvonne Wu is a Ph.D. candidate at Brien Holden Vision Institute and the School of Optometry and Vision Science at the University of New South Wales, Sydney, Australia.
1. Barr J. 2004 Annual Report. CL Spectrum. 2005 Jan. Available at:
www.clspectrum.com/article.aspx?article=12733. (Accessed March 2011).
2. Szczotka-Flynn L, Pearlman E, Ghannoum M. Microbial Contamination of Contact Lenses, Lens Care Solutions, and Their Accessories: A Literature Review. Eye Contact Lens. 2010 Mar;36(2):116-129.
3. Gray TB, Cursons RT, Sherwan JF, Rose PR. Acanthamoeba, bacterial, and funga contamination of contact lens storage cases. Br J Ophthalmol. 1995 Jun;79(6):601-5.
4. Devonshire P, Munro FA, Abernethy C, Clark BJ. Microbial contamination of contact lens cases in the west of Scotland. Br J Ophthalmol. 1993 Jan;77(1):41-5.
5. Yung MS, Boost M, Cho P, Yap M. Microbial contamination of contact lenses and lens care accessories of soft contact lens wearers (university students) in Hong Kong. Ophthalmic Physiol Opt. 2007 Jan;27(1):11-21.
6. Willcox MD, Carnt N, Diec J, et al. Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci. 2010 Jul;87(7):456-64.
7. Wu YT, Zhu H, Harmis NY, et al. Profile and frequency of microbial contamination of contact lens cases. Optom Vis Sci. 2010 Jan;87:152-8.
8. Radford CF, Woodward EG, Stapleton F. Contact lens hygiene compliance in a University population. J Br Contact Lens Assoc. 1993;16:105-11.
9. Mayo MS, Schlitzer RL, Ward MA, et al. Association of Pseudomonas and Serratia corneal ulcers with use of contaminated solutions. J Clin Microbiol. 1987 Aug;25(8):1398-400.
10. Rosenthal RA, Stein JM, McAnally CL, Schlech BA. A comparative study of the microbiologic effectiveness of chemical disinfectants and peroxide neutraliser systems. CLAO J. 1995 Apr;21(2):99-110.
11. McLaughlin-Borlace L, Stapleton F, Matheson M, Dart JKG. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998 May;84(5):827-38.
12. Larkin DF, Kilvington S, Easty DL. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br J Ophthalmology. 1990 Mar;74(3):133-5.
13. Hall BJ, Jones L. Contact Lens Cases: The Missing Link in Contact Lens Safety? Eye Contact Lens. 2010 Mar;36(2):101-5.
14. Simmons PA, Edrington TB, Hsieh L, Wang L. Bacterial contamination rate of soft contact lens cases. International Contact Lens Clinic. 1991 Sep;18(9):188-91.
15. Velasco J, Bermudez J. Comparative study of the microbial flora on contact lenses, in lens cases, and in maintenance liquids. International Contact Lens Clinic. 1996 Mar-Apr;23(2):55-8.
16. Zhang S, Ahearn DG, Noble-Wang JA, et al. Growth and survival of Fusarium solani-F. oxysporum complex on stressed multipurpose contact lens care solution films on plastic surfaces in situ and in vitro. Cornea. 2006 Dec;25(10):1210-6.
17. Ahearn DG, Zhang S, Stulting RD, et al. Fusarium keratitis and contact lens wear: facts and speculations. Med Mycol. 2008 Aug;46(5):397-410.
18. Margolis TP, Whitcher JP. Fusarium—A new culprit in the contact lens case. JAMA. 2006 Aug;296(8):985-7.
19. Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002 May;86(5):536-42.
20. Bates AK, Morris RJ, Stapleton F, et al. ‘Sterile’ corneal infiltrates in contact lens wearers. Eye(Lond). 1989;3(Pt.6):803-10.
21. Stapleton F, Dart JK, Seal DV, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995 Jun;114(3):395-402.
22. Stapleton F, Dart J, Minassian D. Non-ulcerative complications of contact lens wear; relative risks for different lens types. Arch Ophthalmol. 1992 Nov;110(11):1601-6.
23. Wilson LA, Sawant AD, Simmons RB, Ahearn DG. Microbial contamination of contact lens storage cases and solutions. Am J Ophthalmol. 1990 Aug;110(2):193-8.
24. Dart J. The inside story: why contact lens cases become contaminated. Cont Lens Anterior Eye. 1997;20(4):113-8.
25. Lakkis C, Fleiszig SM. Resistance of Pseudomonas aeruginosa isolates to hydrogel contact lens disinfection correlates with cytotoxic activity. J Clin Microbiol. 2001 Apr;39(4):1477-86.
26. Farber BF, Hsieh HC, Donnenfeld ED, et al. A novel antibiofilm technology for contact lens solutions. Ophthalmology. 1995 May;102(5):831-6.
27. Szczotka-Flynn LB, Imamura Y, Chandra J, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009 Sep;28(8):918-26.
28. ISO. Ophthalmic optics-contact lens care products. Microbiological requirements and test methods for products and regimens for hygienic management of contact lenses. Geneva, Switzerland: International Organization for Standardization; 2001.
29. Guidance for industry premarket notification [510(k)]. Guidance document for contact lens care products: Food and Drug Administration; 1997.
30. Wu YT, Zhu H, Willcox M, Stapleton F. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci. 2010 Dec;51(12):6239-33.
31. Kilvington S, Huang L, Kao E, Powell CH. Development of a new contact lens multipurpose solution: Comparative analysis of microbiological, biological and clinical performance. J Optom. 2010;3(3):134-42.
32. Wilson LA, Sawant AD, Ahearn DG. Comparative efficacies of soft contact lens disinfectant solutions against microbial films in lens cases. Arch Ophthalmol. 1991 Aug;109(8):1155-7.
33. Stapleton F. Contact lens related microbial keratitis and bacterial biofilm: an overview. Optician. 1990;199:17-20.
34. Wu Y, Carnt N, Willcox M, Stapleton F. Contact lens and lens storage case cleaning instructions: whose advice should we follow? Eye Contact Lens. 2010 Mar;36(2):68-72.
35. Amos CF, George MD. Clinical and laboratory testing of a silver-impregnated lens case. Cont Lens Anterior Eye. 2006 Dec;29(5):247-55.
36. Collins MJ, Carney LG. Patient compliance and its influence on contact lens wearing problems. Am J Optom Physiol Optic. 1986 Dec;63(12):952-6.
37. Wu Y, Carnt N, Stapleton F. Contact lens user profile, attitudes and level of compliance to lens care. Cont Lens Anterior Eye. 2010 Aug;33(4):183-8.
38. Hickson-Curran S, Chalmers R, Sencer S. Making the case for daily disposable contact lenses: patient non-compliance with storage case hygiene and replacement. Presented at the American Academy of Optometry meeting, Nov 2010; San Francisco.
39. Mayers M, Callan B, Borazjani R. Compliance and contamination in contact lens wear. After American Academy of Optometry meeting, 2010; San Francisco.
40. Woods C, Dumbleton K, Richter D. Compliance with lens care and contact lens case care and replacement. American Academy of Optometry meeting, 2010; San Francisco.
41. Contact Lens London: Look after your eyes; The College of Optometrists 2009. Available at:
http://lookafteryoureyes.org/en/eye_wear/contact_lenses.cfm (Accessed March 2011).
42. Larragoiti ND, Diamos ME, Simmons PA, Edrington TB. A comparative study of techniques for decreasing contact lens storage contamination. J Am Optometric Assoc. 1994 Mar;65(3):161-3.
43. Hiti K, Walochnik J, Faschinger C, et al. Microwave treatment of contact lens cases contaminated with acanthamoeba. Cornea. 2001 Jul;20(5):467-70.
44. Mowrey-McKee MF, Monnat K, Sampson HJ, et al. Microbial contamination of hydrophilic contact lenses. Part I: Quantitation of microbes on patient worn-and-handled lenses. CLAO J. 1992 Apr;18(2):87-91.
45. Mowrey-McKee MF, Sampson HJ, Proskin HM. Microbial contamination of hydrophilic contact lenses. Part II: Quantitation of microbes after patient handling and after aseptic removal from the eye. CLAO J. 1992 Oct;18(4):240-4.
46. Pittet D, Dharan S, Touveneau S, et al. Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med. 1999 Apr;159(8):821-6.
47. Levy B, Heiler D, Norton S. Report on testing from an investigation of fusarium keratitis in contact lens wearers. Eye Contact Lens. 2006 Dec;32(6):256-61.
48. Stapleton F, Dart JKG, Minassian D. Risk factors in contact lens related suppurative keratitis. CLAO J. 1993 Oct;19(4):204-10.
49. Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008 Oct;115(10):1655-62.
50. Wu YT, Zhu H, Willcox M, Stapleton F. Impact of air-drying lens cases in various locations and positions. Optom Vis Sci. 2010 Jul;87(7):465-8.
51. FDA. Focusing on contact lens safety. 2008 Nov. Available at:
www.fda.gov/ForConsumers/ConsumerUpdates/ucm048893.htm (Accessed March 2011).
52. Lakkis C, Anastasopoulos F, Terry C, Borazjani R. Time course of the development of contact lens case and contact lens contamination. Abstract presented at the Association for Research in Vision and Ophthalmology meeting, 2009; Ft Lauderdale, FL.
53. Sokol JL, Mier MG, Bloom S, Asbell PA. A study of patient compliance in a contact lens-wearing population. CLAO J. 1990 Jul-Sep;16(3):209-13.
54. De Oliveira PR, Temporini-Nastari ER, Ruiz Alves M, Kara-José N. Self-evaluation of contact lens wearing and care by college students and health care workers. Eye Contact Lens. 2003 Jul;29(3):164-7.
55. Shapiro DE, Boggs SR, Melamed BG, Graham-Pole J. The effect of varied physician affect on recall, anxiety, and perceptions in women at risk for breast cancer: an analogue study. Health Psychol. 1992;11(1):61-6.
56. Kessels RP. Patients’ memory for medical information. J R Soc Med. 2003 May;96(5):219-22.
57. Houts PS, Witmer JT, Egeth HE, et al. Using pictographs to enhance recall of spoken medical instructions II. Patient Educ Couns. 2001 Jun;43(3):231-42.
58. Bui TH, Cavanagh HD, Robertson DM. Patient Compliance During Contact Lens Wear: Perceptions, Awareness, and Behavior. Eye Contact Lens. 2010 Nov;36(6):334-9.
59. Carnt N, Keay L, Willcox M, et al. Higher risk taking propensity of contact lens wearers is associated with less compliance. Cont Lens Anterior Eye. 2010 Nov. [Epub ahead of print]
60. Cook PA, Bellis MA. Knowing the risk: relationships between risk behaviour and health knowledge. Public Health. 2001 Jan;115(1):54-61.
61. Bowden T, Harknett T. Contact lens wearer profile 2004. Contact Lens Ant Eye. 2005 Mar;28(1):37-45.


