According to a recent study in Cornea, approximately one million annual ocular medical visits in the United States ended up with a bacterial keratitis diagnosis.1,2 Of these patients, 76.5% received a prescription for antibiotics from their healthcare provider.1,2
The cost of treating bacterial keratitis is estimated to be around $377 million to $857 million per year.3 Bacterial keratitis is only one cause of ocular infections, but it requires immediate intervention in order to prevent vision loss and minimize complications.
Viral and bacterial infections are the most common etiologies for bacterial keratitis. A proportion of 71,000 cases of severe infectious keratitis a year in America has been estimated, a lower proportion than non-infectious bacterial keratitis.2,4
To treat bacterial keratitis, practitioners initiate empirical therapy with broad-spectrum or fortified antibiotics prescriptions; however, overuse has led to a pattern of resistance that can cause difficulty in suitably managing the condition.5
This article explores the changes in trends regarding corneal infections and the situations where practitioners should use antibiotic treatment. Understanding common pathogens and effective treatments is essential in managing these patients with the most successful results.
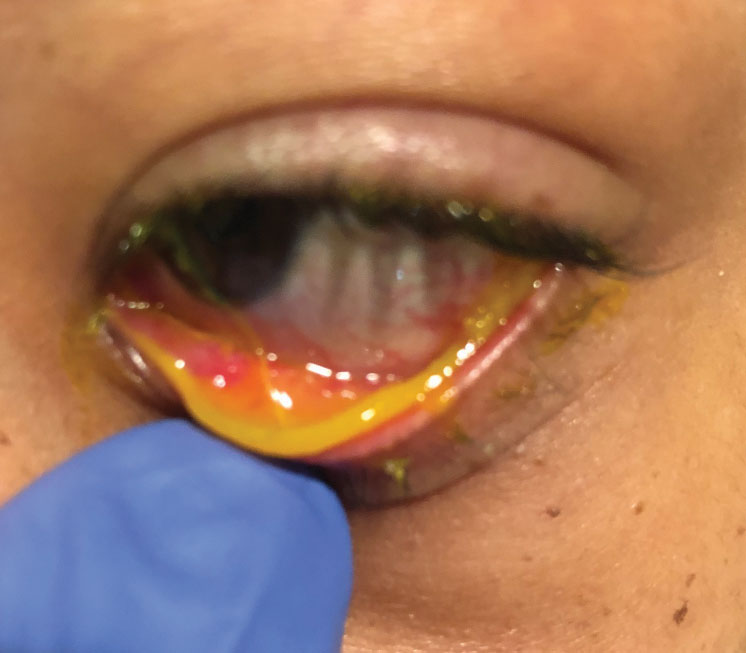 |
| This child has subcutaneous conjunctival membranes from a bacterial infection. Click image to enlarge. |
Bacterial Conjunctivitis and Keratitis
Bacterial conjunctivitis is the second most common cause of conjunctivitis, and it is responsible for 50% to 75% of conjunctivitis cases in children.6 Although conjunctivitis involves the conjunctiva specifically, it can affect the surrounding ocular structures that can lead to worsening infections, such as keratitis, which can become serious enough to cause blindness.7
In adults, a bacterial origin is less common than a viral one and is characterized by bacterial overgrowth, along with infiltration of the conjunctival epithelial layer. The origin can either be from direct contact with an infected individual’s secretions or advanced through organisms colonizing within the patient’s own nasal and sinus mucosa.8
Bacterial keratitis is an acute or chronic condition that can become sight-threatening if left untreated. These cases can lead to stromal inflammation and progressive tissue destruction, eventually causing perforation. It is commonly connected with risk factors that disturb the corneal epithelial integrity. Contact lens wear, trauma, impaired defense mechanism, immunosuppressive medication use and altered corneal surface structure postoperatively are all common predisposing factors.8,9
The most common risk factor in the US is contact lens wear. Microbial keratitis is approximately 15 times more likely in patients who sleep in their lenses and is positively correlated with the number of days patients wear their contact lenses without removal.8 With the increase of contact lens wear gloally, bacterial keratitis has also increased accordingly.9
Bacterial conjunctivitis can be self-limiting and resolves on its own in one to two weeks due to the body’s immune factors.6 Gram-positive cocci and Staphylococcus species are known to inhabit skin cells, skin glands and mucous membranes. Methicillin-resistant Staphylococcus aureus (MRSA) is a well-known gram positive cocci we always have to be on the lookout for. MRSA has a caused an increase of vancomycin use. Propionibacterium acnes (P. acnes) is one of the major causes of postoperative endophthalmitis but an uncommon cause of microbial keratitis.
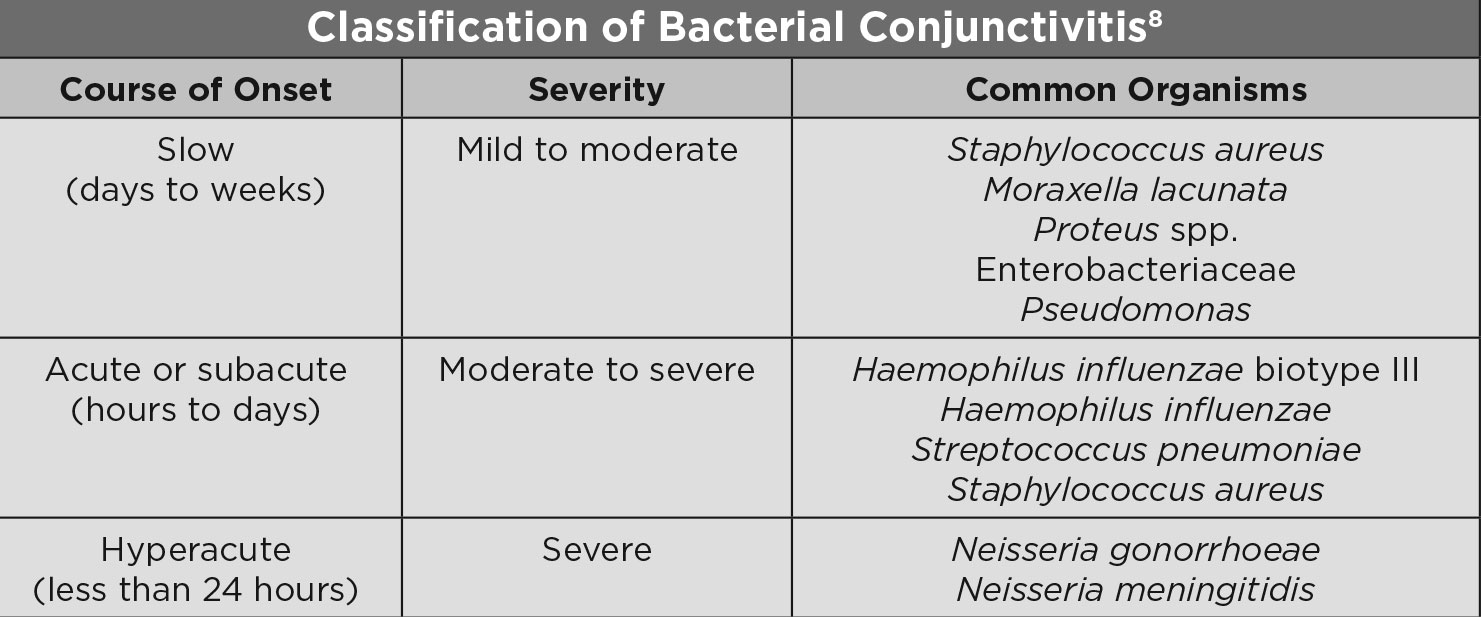 |
| Classification of Bacterial Conjunctivitis. Click table to enlarge. |
When caused by dangerous bacterial species, such as Neisseria gonorrhoeae or Streptococcus pyogenes, bacterial conjunctivitis can be serious and sight-threatening. In rare cases, it may foreshadow a life-threatening systemic disease, such as conjunctivitis caused by Neisseria meningitidis.8
The most common causative bacterial species are Haemophilus influenza, Streptococcus pneumoniae, Staphylococcus aureus and Staphylococcus epidermidis with staphylococci, specifically S. aureus and coagulase-negative Staphylococci (CoNS), reported most often.7,10
Several bacterial species simultaneously can cause most cases of acute bacterial conjunctivitis. This is why practitioners use broad-spectrum antibiotics as the first line of ophthalmic antibacterial treatment.1 Antibiotics often accelerate clinical resolution and microbiological remission, while also lessening the risk of recurrence and the development of complications.1
Some prospective studies show that delaying antibiotic treatment until day three or four will reduce the use of unnecessary medications and not affect outcomes. The practitioner only initiated treatment if the signs were worsening, shortening the course and improving symptoms. These studies all advocate that initiation of antibiotics after day four provides finite benefits.8
Classic antibacterial options include tobramycin, trimethoprim, ciprofloxacin, gatifloxacin and moxifloxacin.10 However, the widespread use of broad-spectrum antibiotics has resulted in resistance to those typical antibiotics.10 Therefore, developing new antibiotics with high efficacy and safety against some resistant bacteria is necessary.1
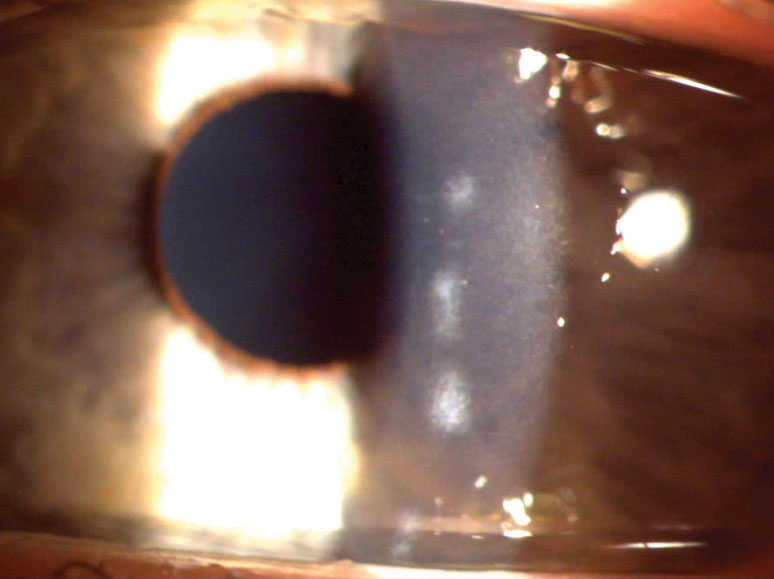 |
| This patient had bacterial keratitis (top) that eventually resolved with antibiotics and was left only with a small scar (bottom). Click images to enlarge. |
 |
Management
The primary goal when dealing with corneal infections is always to prevent loss of sight and to preserve corneal clarity. It is safer to assume and, therefore, treat any presentation of microbial keratitis as bacterial keratitis for the best outcome.9 However, it is difficult for the practitioner to quickly and effectively manage patients with presumed microbial conjunctivitis or keratitis. Although it would be ideal to have a confirmed definitive diagnosis before initiating therapy, bacterial pathogens can cause irreversible corneal scarring. It is therefore imperative to begin treatment before any damage occurs. The initiation of therapy must occur before an established diagnosis in order to prevent visual disability and limit the bacterial load.8
Preliminary therapy is comprised of empirical topical broad-spectrum antibiotics. For routine corneal ulcers, monotherapy of topical fluoroquinolones provides comparable therapy to combination therapy due to the enhanced penetration obtained with commercially available fluoroquinolones.8 Fluoroquinolones can be instilled every 30
minutes to 60 minutes for a routine corneal ulcer.
If the ulcer is more severe, use a loading dose of every five minutes for 30 minutes to transfer therapeutic concentration to the stroma faster.8
If monotherapy fails and/or the initial ulcer is large, central or atypical, consider combination therapies due to the additional gram-negative activity. In addition, if combination therapy fails or MRSA is suspected, initiate fortified antibiotics, including vancomycin. Fortified antibiotics are compounded at increased concentration. Remember that “fortifieds” can be difficult to obtain commercially and have greater corneal toxicity. According to a survey, the majority of corneal specialist respondents in the United States chose to treat corneal ulcers with fortified antibiotics, specifically vancomycin and tobramycin due to low antibiotic resistance, whereas a majority of international corneal specialists respondents chose fluoroquinolone treatment due to availability.11
Clinical parameters that can be helpful to monitor the response to antibiotic treatment include blunting the stromal infiltrate perimeter, decreased density of the stromal infiltrate, reduction of stromal edema and endothelial inflammatory plaque, reduction in anterior chamber inflammation, reepithelization and cessation of corneal thinning.8
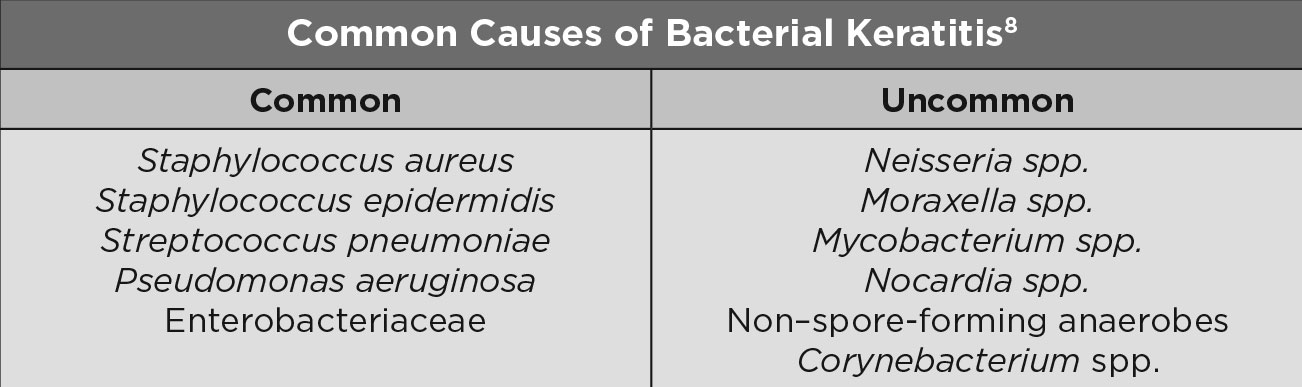 |
| Common Causes of Bacterial Keratitis. Click table to enlarge. |
In day-to-day clinical practice, we work side-by-side with anterior segment specialists in a tertiary care setting. Over the years, we have developed our own “smart” strategies for dealing with corneal infections.
- Take a careful case history. Do not rely only on the information the patient gives you. Always ask about previous contact lens use, recent activities, surgeries, etc.
- Ask about previous treatments. Many patients have already gone to a walk-in clinic or someone who is not an eye care provider. If they can’t recall the exact medication given, ask about the cap color and dosage.
- Conduct a careful slit lamp exam including lid eversion and fluorescein staining.
- Use slit lamp photographs to compare images at each visit.
- Perform IOP measurements and dilation are essential. If you haven’t seen a patient recently, you cannot assume the posterior segment is not involved.
Once the presumed etiology is determined based on case history and clinical exam, personal experience shows it is most effective to use monotherapy of topical fluoroquinolones during the day and an added ointment at night. In the office, fourth generation fluoroquinolones are not always available; therefore, we give the patient a sample of the highest-generation drop available and a prescription for fourth generation fluoroquinolones to the pharmacy.
Depending on the severity of the infection, it is helpful to space out follow-ups in order to give the antibiotics time to work and the cornea time to heal. At the follow-up, closely compare the current presentation to previous slit lamp photos. Oftentimes, clinical signs and patient symptoms will have started to improve before culture results have returned.
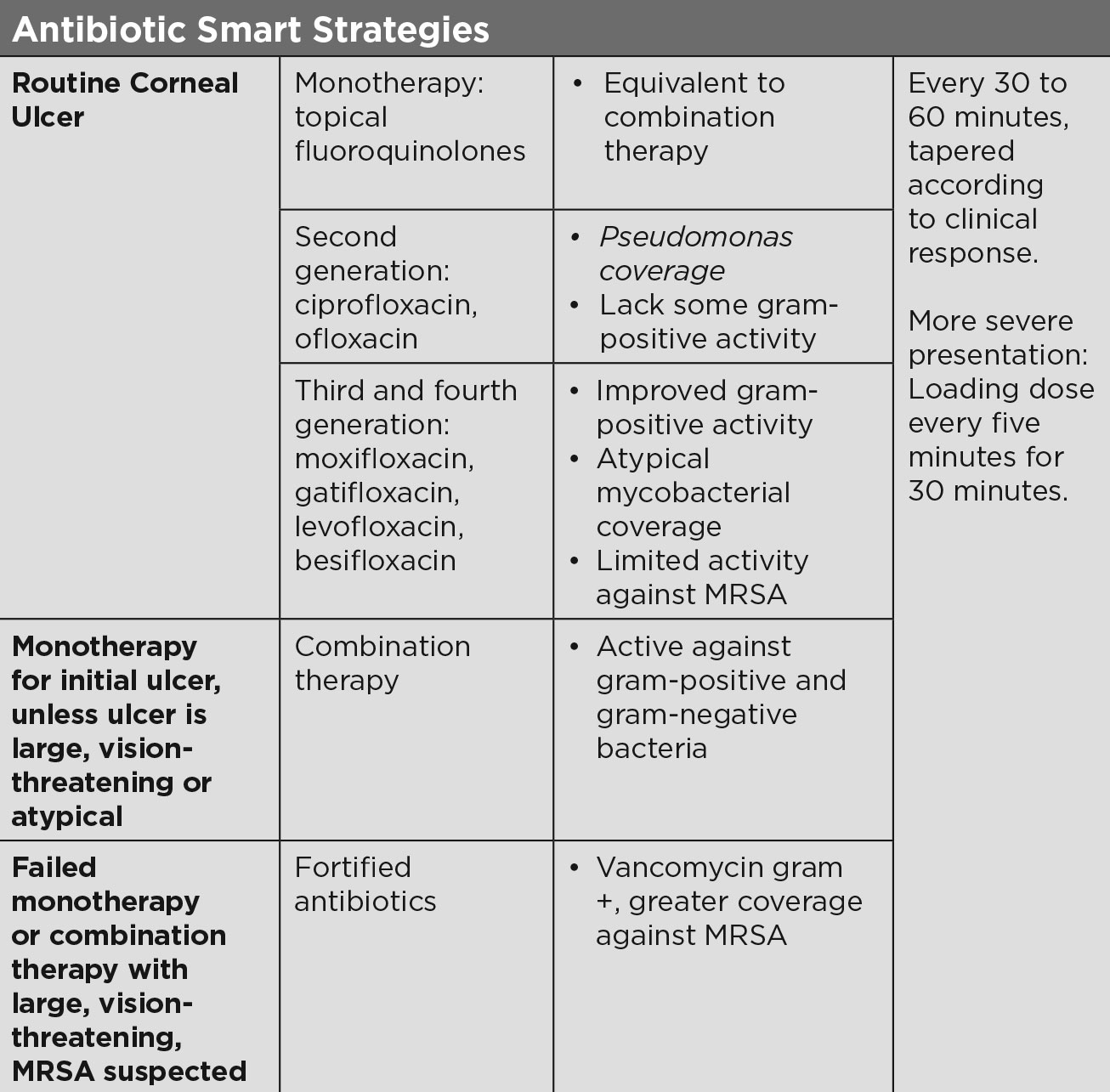 |
| Antibiotic Smart Strategies. Click table to enlarge. |
Rise of the Resistance
Though bacterial keratitis requires treatment with antibiotics, it is crucial to understand how over-using and over-prescribing antibiotics can lead to resistance. Bacterial resistance to an antibiotic depends on the mechanism. The most common resistance mechanism, modification, can involve a mutation to the target site, making the drug ineffective.12 Resistance can be coded into the bacterial genes and then passed between colonies and species, allowing it to spread quickly.12
Antibiotic resistance to penicillin can begin soon after the drug is introduced to treat infections.13 Factors to blame for antibiotic resistance include over-prescribing, inappropriate dosing regimen, increased use of antibiotics in agriculture and increased exposure to systemic antibiotics.14,15 When practitioners prescribe, a pattern of resistance can occur if patients are unable to self-administer properly or discontinue medications due to ocular discomfort from adverse effects.15
Many of the antibiotics treating the ocular surface are also used systemically for infections, except besifloxacin, which was formulated exclusively for ocular use to allow lower resistance rates.14 We have seen some patients who believe they are cured and self-discontinue antibiotics early. Once this happened, the infection reappeared and the treatment course had to be resumed. It is therefore wise for optometrists to prevent over-prescribing and make sure the antibiotic treatment runs its course.
The increase in resistant bacteria over the years has led to studies, such as the Ocular Tracking Resistance in the United States Today (TRUST) and Antibiotic Resistance Monitoring in Ocular micRoorganisms (ARMOR) studies.13,14 Ocular TRUST monitored S. aureus, S. pneumoniae and H. influenzae when treated with fluoroquinolones, macrolides, aminoglycosides, penicillin, dihydrofolate reductase inhibitors and polypeptides. Ocular TRUST studies reported 16.8% methicillin resistance from 2005 to 2006, which then increased to 50% by 2008.16
The ARMOR study monitors antibiotic resistance in ocular infections against S. aureus, CoNS, S. pneumoniae, H. influenzae and P. aeruginosa.16 From 2009 to 2013, methicillin resistance was shown in staphylococcal isolates; however, it did not increase over the five years. Bacteria such as S. pneumoniae and H. influenzae were most susceptible to antibiotics, whereas there was multidrug resistance in 86.8% of methicillin-resistant S. aureus isolates and 77.3% of methicillin-resistant CoNS. The study also noted a higher number of methicillin-resistant staphylococcal infections in elderly patients.16 P. aeruginosa and H. influenzae isolates showed low resistance against most of the antibiotics tested.
Most of the published data regarding ocular pathogens is collected from single centers; however, pathogens differ in prevalence geographically, reinforcing the need for studies like ARMOR, which are conducted nationwide in the United States. Understanding the location of bacteria can aid eyecare providers to target the more common pathogens—S. aureus is higher in the South and lower in the West, S. pneumoniae is higher in the Midwest and lower in the West and P. aeruginosa is higher in the Midwest and lower in the West.
Globally, antibiotic resistance continues to plague practitioners when treating keratitis. Moxifloxacin has shown increased resistance in India, despite its more “recent” foray in treating bacterial infections; there was low susceptibility of coagulase-negative Staphylococcus (61.2%) and methicillin-resistant Staphylococcus (53.1%) when treated with moxifloxacin. In the US, 26% of all organisms cultured were resistant to moxifloxacin, while 28% of Staphylococcus aureus bacterial isolates were resistant to ciprofloxacin or ofloxacin.17
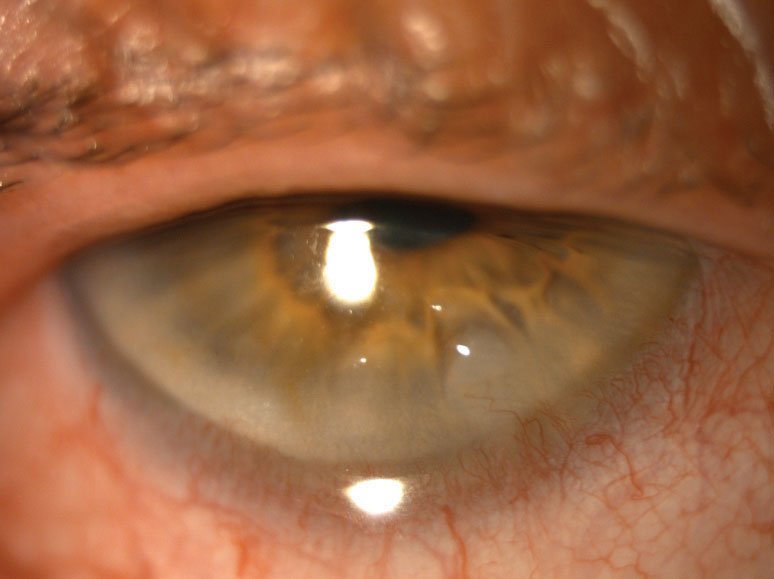
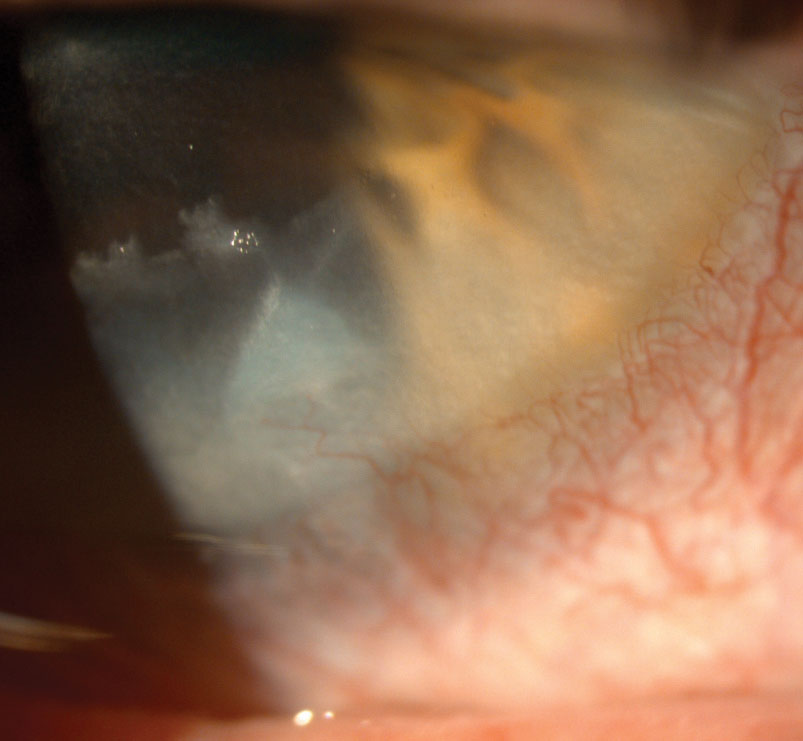 |
| Corneal scarring secondary to chronic blepharoconjunctivitis caused this case of keratitis. Click image to enlarge. |
Alternative Therapy
There are other treatment modalities to consider when treating bacterial keratitis. The use of steroids in treating bacterial keratitis remains a controversial issue. Early use of steroids can help reduce corneal stromal melt, neovascularization and corneal scarring that results from the inflammatory response against the infection. Instilling steroids in conjunction with fortified antibiotics can also help decrease discomfort. The counterargument is that steroids delay corneal healing, leading to a worse infection. Though steroid use leads to improvement for some patients, note that the use of steroids for fungal or Acanthamoeba infections can lead to terrible results, such as increased loss of vision or loss of the eye.
Studies have been performed to find new adjunct therapies to treat bacterial keratitis, especially with the rise of antibiotic resistance. Amniotic membranes, typically used during pterygium surgery, also help resolve epithelial defects and chemical injury. Amniotic membrane benefits include reepithelization by reinforcing basal epithelial cell adhesion, induction of epithelial cell migration, differentiation and proliferation of conjunctival and limbal epithelial progenitor cells, prevention of epithelial apoptosis and reduction of keratocyte apoptosis. Studies have shown improvement in epithelial defect, corneal haze and neovascularization when eyes were treated with amniotic membranes rather than antibiotics alone; however, larger studies needs to be conducted to analyze the full potential of amniotic membranes when treating bacterial keratitis.18
Treatment for corneal perforation with doxycycline has shown improvement in animal studies. In rabbit models, corneal perforation from Pseudomonas ulcers was reduced by 50% with systemic doxycycline use. Unfortunately, the lack of human studies makes it difficult to prove doxycycline as an effective therapy.
Collagen crosslinking, used to treat keratoconus, has antimicrobial properties and can potentially help resolve corneal ulcers from bacterial pathogens. Case reports have shown an improvement in symptoms and the resolution of treatment-resistant infections. Further trials and studies could help establish crosslinking as a viable treatment for those with antibiotic resistance and/or to prevent ocular toxicity.19
Though bacterial keratitis needs to be treated aggressively, exercise caution when using treating topical therapy options, such as steroids. Clinical assessment is imperative in making the correct diagnosis and managing it appropriately prevents vision loss.
Dr. Shuja is an optometrist at New York–Presbyterian Hospital.
Dr. Sherman is an assistant professor of optometric sciences in ophthalmology and director of optometric services at the Columbia University Medical Center and an assistant attending at New York–Presbyterian Hospital.
1. Collier SA, Gronostaj MP, MacGurn AK, et al. Estimated burden of keratitis: United States, 2010. Morb Mortal Wkly Rep. 2014;63(45):1027-30. 2. Ballouz D, Maganti N, Tuohy M, et al. Medication burden for patients with bacterial keratitis. Cornea. 2019;38(8):933–7. 3. Smith AF, Waycaster C. Estimate of the direct and indirect annual cost of bacterial conjunctivitis in the United States. BMC Ophthalmol. 2009;9:13. 4. Jeng BH, Gritz DC, Kumar AB, et al. Epidemiology of ulcerative keratitis in Northern California. Arch Ophthalmol. 2010;128:1022–8. 5. Gokhale NS. Medical management approach to infectious keratitis. Indian J Ophthalmol. 2008;56(3):215-20. 6. Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86(1):5-17. 7. Teweldemedhin M, Gebreyesus H, Atsbaha AH, et al. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmology. 2017;17:212. 8. American Academy of Ophthalmology. External disease and cornea: Basic and Clinical Science Course 2017-2018. 2017. 9. Wang JJ, Gao XY, Li HZ, Du SS. The efficacy and safety of besifloxacin for acute bacterial conjunctivitis: a Meta-analysis. Int J Ophthalmol. 2019;12(6):1027-36. 10. Al-Mujaini A, Al-Kharusi N, Thakral A, Wali UK. Bacterial keratitis: perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ Med J. 2009;9(2):184–95. 11. Austin A, Schallhorn J, Geske M, et al. Empirical treatment of bacterial keratitis: an international survey of corneal specialists. BMJ Open Ophthalmol. 2016;2:e00047. 12. Baum J, Barza M. The evolution of antibiotic therapy for bacterial conjunctivitis and keratitis: 1970-2000. Cornea. 2000;19(5):659-72. 13. McDonald M, Blondeau J. Emerging and antibiotic resistance in ocular infections and the role of fluoroquinolones. J Cataract Refract Surg. 2010;36(9):1588-98. 14. Thomas RK, Melton R, Asbell PA. Antibiotic resistance among ocular pathogens: current trends from the ARMOR surveillance study (2009-2016). Clin Optom (Auckl). 2019;11:15-26. 15. Samarawickrama C, Chan E, Daniell M. Rising fluoroquinolone resistance rates in corneal isolates: implications for the wider use of antibiotics within the community. Infection, Disease & Health. 2015;20:128-33. 16. Asbell PA, Sanfilippo CM, Pillar CM, et al. Antibiotic resistance trends from ocular pathogens in the US-cumulative results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. US Ophthalmic Review. 2017;10(1):35-8. 17. Ung L, Bispo PJM, Shanbhag SS, et. al. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64:255-71 18. Dakhil TAB, Stone DU, Gritz DC. Adjunctive therapies for bacterial keratitis. Middle East Afr J Ophthalmol. 2017;24(1):11–7. 19. Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124(11):1678–89. |


