Not too long ago, the contact lens marketplace seemed a bit stagnant. Practitioners had a stable line-up of offerings that served patients well, but it was essentially the same product lines year in and year out with some incremental updates. For 2019, however, industry has some ambitious new ideas to debut. Here, we offer a sneak peek of what’s expected in the coming months, and optometrists weigh in on how these new lenses might fit into what’s currently available.
“In general, the lenses will be a stab at what doctors and patients have been asking for over the last several years,” says David Anderson, OD, of Miamisburg, Ohio. “We will see toric multifocals, lenses to treat medical conditions and lenses that change color like Transitions glasses. For the longest time, the lens advances have been all health driven, addressing compliance with daily disposables or more oxygen with silicone hydrogel lenses. Now, the companies are making an effort to focus on the cosmetic and medical arenas to help solve some needs that patients have had for years.”
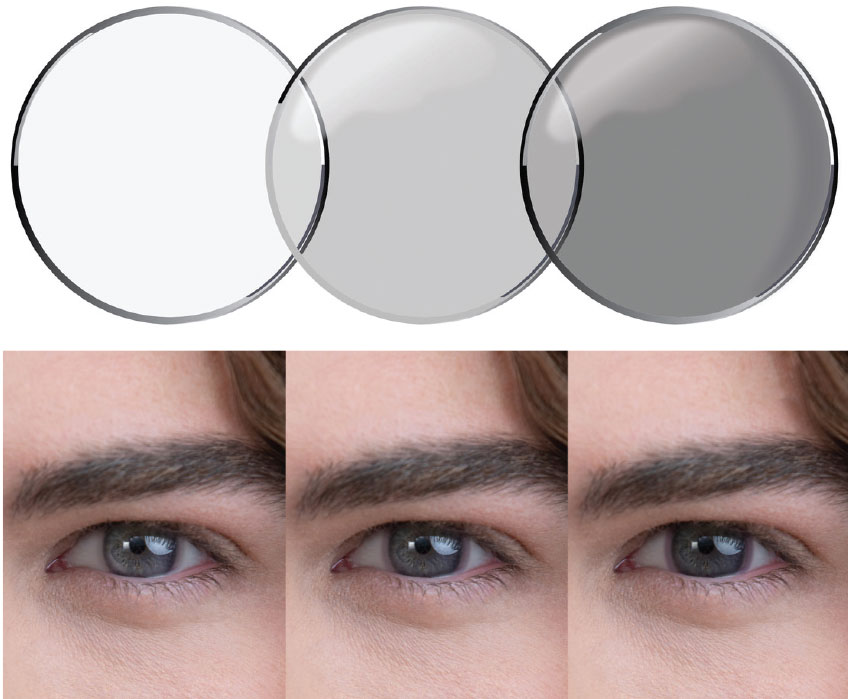 |
| Fig. 1. and Fig. 2. The Acuvue Oasys with Transitions Light Intelligent Technology lenses become dark in 45 seconds when exposed to bright light and fade back to clear within 90 seconds in darker lighting, according to the company. |
Sun Protection
For the first time, photochromic technology is coming to the contact lens platform, with a new lens under the Transitions banner from Johnson & Johnson Vision (JJV) slated to hit the market in the first half of this year. Formally named Acuvue Oasys with Transitions Light Intelligent Technology, the lens is a two-week reusable product that continuously adapts from clear to dark and back, according to the company. The lenses become dark in 45 seconds when exposed to UV or HEV light and fade back to clear within 90 seconds in darker lighting. The lens also provides 100% protection against UVB rays, JJV says. The lens is the result of a joint partnership between JJV and Transitions Optical.
“This lens has been long awaited for,” says Dr. Anderson. “The idea of both protecting your eyes from UV and shielding them from the sun’s brightness without carrying sunglasses has been on patients’ minds for some time. Specifically, at least once a month for as long as I have been in practice, I’ve gotten the question about photochromic contact lenses. There is clearly a desire, and I am excited to answer ‘yes’ to this question several times a year, and possibly more, as awareness increases once the product is available.”
Mile Brujic, OD, of Bowling Green, Ohio, likens this new lens option to a “sunglass contact lens.” Although it doesn’t provide ocular tissue protection, athletes who may not be able to wear sunglasses, for example, will be able to see comfortably in this new lens. “In my mind, that’s the bigger thing than the actual protective factors of the potential UV protection in the lens.”
Adds Glenda Secor, OD, of Huntington Beach, CA, “This lens should be great. Patients are anxious to try them.”
Are CL Developments Meeting ODs’ Needs?We asked experts from the Centre for Ocular Research & Education (CORE) at the University of Waterloo to weigh in on whether the new contact lenses being developed are bridging doctor and patient needs. At a basic level, contact lenses need to enable patients to see clearly, with all-day comfort, while maintaining the health of the wearer’s eye, they say. For practitioners, lenses need to be quick and predictable to fit. “Many changes in contact lens design and technology over the last few years have helped us move closer to being able to provide those basics for a greater number of patients,” they say. For example, silicone hydrogel materials provide the cornea with sufficient oxygen for daily wear, and, in many cases, adequate oxygen for overnight wear also. Materials have been engineered to try and maintain comfort throughout the day, and choice of design to correct astigmatic and presbyopic prescriptions has increased. Yet, there are still improvements to be gained in delivering enhanced comfort for the many patients who experience contact lens-related dryness, they add. “We need to understand more about the interaction of contact lenses with both the tear film and contact lens solutions, and we would benefit from technology which enables the incidence of both microbial keratitis and corneal infiltrative events to be reduced.” Acquisitions of instrument and therapeutic device intellectual property by some of the major vision care companies over the past few years demonstrate the focus that exists in this area, they add. “A better understanding of the tear film and how it interacts with the contact lens should lead to new technology and treatments which can help maintain, or even enhance, ocular surface health,” the CORE researchers explain. |
New Presbyopic Options
Last fall, Alcon launched its monthly replacement Air Optix plus HydraGlyde Multifocal contact lens, giving the company a new monthly option to round out its multifocal product line. The HydraGlyde component is said to improve moisture retention and thus contact lens comfort, according to the company.
Other companies are also planning new multifocal offerings for this patient population:
Astigmatism. Bausch + Lomb is gearing up to debut a multifocal toric lens, the Ultra Multifocal for Astigmatism. The monthly replacement silicone hydrogel lens can correct near or distance vision, astigmatism and presbyopia and has an expected release of mid-2019.
This lens may help patients with uncorrected astigmatism stay in contact lenses because they won’t need to wear readers over their distance vision contacts or rely on monovision to help with near vision, says Dr. Anderson. “A multifocal option to also correct astigmatism offers an opportunity to help more patients as they lose their near vision, and provides an option as many patients have an increase in astigmatism as they age, which alone can cause dropout,” Dr. Anderson adds.
“This is the first time we’ve had access to a toric contact lens that has presbyopia-correcting options available in our office without having to specialty order them,” says Dr. Brujic.
While a multifocal for astigmatism has existed for many years, it is a custom-fit lens, which presents myriad clinical and logistical barriers. “The process is lengthy because it is a made-to-order lens,” says Justin Bazan, OD, of Park Slope Eye in Brooklyn, NY. “It can take a couple of weeks just to get the first trial lenses. More times than not, a second or third trial is needed, adding several weeks to complete the fit. This drawn-out process often leads to frustration and many patients are lost to follow-up.”
Also, the fitting process for custom lenses is complicated and the visual outcome isn’t always satisfactory, Dr. Bazan says. “For me, the complication stems from numerous add powers and power designs. It’s rare to get a patient who is happy with their visual outcome.”
The forthcoming lens from B+L may help to resolve these issues, Dr. Bazan says. “It combines technology that has been proven successful, it is easy to fit and will be available on the spot in an in-office fitting kit. From our spherical presbyopic patients, we have learned that multifocal contact lenses are the preferred way to handle presbyopia, and now we are finally able to offer it to our astigmatic presbyopic patients.”
“It will be like fitting a spherical multifocal lens, and the Ultra material is a comfortable platform,” Dr. Brujic adds. “This is one I’m excited about.”
Dynamic refraction. Presbyopic patients may one day have access to a self-powered smart lens designed to dynamically change focus. The subject of much speculation for years, this concept is being pursued by at least two companies, Alcon and JJV. Both have publicly discussed their plans to develop a lens that adjusts its shape to change the refractive index as needed. JJV says it has overcome many technical hurdles, including onboard battery technology, and it is working closely with the FDA on the regulatory pathway for their innovation. An accommodating contact lens still appears to be on Alcon’s product roadmap, too, according to public documents.
Though a product launch won’t make it into 2019, clinicians may be able to hear more about these technologies as plans proceed at both companies.
A Mid-tier SiHy Lens
Back in 2011, Alcon planted a flag at the high end of the daily disposable market with the launch of its DailiesTotal1 silicone hydrogel (SiHy) lens, using a water gradient lens matrix. Subsequent product line extensions added correction for astigmatism and presbyopia. The company also has a non-SiHy workhorse lens in the Dailies Aqua Comfort Plus product line.
In 2019 or perhaps 2020, look for the company to add a third category in between those two, reportedly to be called Precision1. Few details are available, but the company expects the silicone hydrogel contact lens to use what it calls ‘advanced aqueous extraction and surface treatment,’ which it believes will help the contact lens compete with other mainstream silicone hydrogel options currently on the market.
In statements to financial analysts, the company said Precision1 “will be a daily disposable, SiHy contact lens intended to compete within the mainstream subcategory of the global daily disposable contact lens market” and it “will be engineered for the highest visual clarity of any contact lens in its class.” Positioned between the high-end Dailies Total1 and the non-SiHy Dailies Aqua Comfort Plus, this new, mid-tier lens could strike a balance between performance and cost that helps grow the daily disposable category as a mass-market product.
Further down the road, we may also see an advance that builds upon the Dailies Total1 lens matrix technology. Investor presentations note a “next generation water gradient” material in the works, though no release date is specified.
Happy 20th, Silicone Hydrogels!By Lyndon Jones, PhD, DSc, FCOptom, Jill Woods, BSc (Hons), MCOptom, Karen Walsh, BSc (Hons), MCOptom, and Doerte Luensmann, PhD, Dipl. Ing. In 2018, silicone hydrogel (SiHy) contact lenses celebrated their 20th birthday. Now that these lenses have been available for two decades, we at CORE offer a walk down memory lane. Here is a look at SiHy’s milestones and challenges, from balancing properties for comfortable daily wear to increased understanding of how the lenses interact with the ocular surface and tear film, plus a glimpse into the future: Infant/Toddler Years: 1998-2003 Prior to SiHy lenses, frequent replacement soft hydrogel lenses were available, but hypoxia-related complications existed with full-time daily and extended wear. The potential benefits of silicone were known, but researchers and manufacturers faced significant technical challenges when incorporating the hydrophobic element into a lens. The first generation of SiHy lenses were balafilcon A, with a plasma oxidation surface treatment that created silicate “islands’ on the surface of the lens, and lotrafilcon A with a plasma coating. Both required surface modification to create a suitably hydrophilic surface. The first years of the SiHy era delivered both a leap forward in oxygen delivery and some initial physiological challenges. Hypoxic responses, such as corneal striae, epithelial microcysts, limbal hyperemia and corneal neovascularization were significantly reduced. However, mechanical complications arose from the combination of increased modulus and original base curve designs. These included contact lens induced papillary conjunctivitis, mucin balls, epithelial splits and discomfort. Elementary Years: 2004-2009 This was a period of great advancements for SiHy with the launch of the first reusable SiHy lens with a daily wear-only indication. This material, galyfilcon A, had a bound internal wetting agent to achieve wettability. Further innovation saw the release of comfilcon A, an inherently wettable material. Practitioners had access to an increased choice of spherical lenses, along with the addition of toric and multifocal options across a number of new materials for both extended and daily wear. The first daily disposable SiHy lens, narafilcon A, was launched in 2008-2009. Balancing the material properties of oxygen transmissibility, modulus, coefficient of friction and wettability was a focus through these years to drive increased comfort for daily wear. Packaging solutions also received attention, with comfort-enhancing agents added to the blister pack of several SiHy materials. However, adverse reactions still occurred, including the potentially sight-threatening complication of microbial keratitis. Additionally, reusable SiHys were found to be two times more likely to result in corneal infiltrative events compared with hydrogel lenses.1-4
High School: 2010-2017 New research and development resulted in an increased understanding of the interaction of SiHys with the tear film. This included establishing protein and lipid deposition profiles, and the relevance of the conformational state of those tear components once adsorbed onto, and absorbed into, the contact lens. Further investigations also explored other variables that may impact comfort, such as the effect of contact lens wear on the ocular inflammatory response and the interactions that occur between SiHy materials and contact lens care systems. Key milestones during this era included a new lens material, delefilcon A, with a silicone core and a hydrogel-like surface, updated designs from the original 1998 lenses, and the emergence of color SiHys. By the end of 2017, more than 40 SiHys were available in all prescriptions and modalities, including daily disposables for astigmatism and presbyopia. By 2017, two-thirds of all soft fits were SiHys, with the greatest use of this material occurring with reusable contact lenses.5 While understanding SiHy materials and their ocular interactions increased during this time, some questions remain and are the subject of ongoing research. Hopefully innovation will result in lenses with reduced complication rates and improved all-day comfort. College Years: 2018 and Beyond Today, researchers are looking into the development of materials to reduce the incidence of infective (microbial keratitis) and inflammatory (corneal infiltrative) events. This may involve the addition of antimicrobial coatings to contact lens materials. In addition, improving comfortable wear times is another goal, and researchers are studying the controlled interaction with the tear film, which would encourage uptake of “good” proteins and lipids while resisting deposition of “bad” tear film components. Investigators believe the conformational state of deposits is important, with materials ideally being able to minimize the denaturation of proteins and oxidation of lipids. Further comfort enhancements may also involve differential deposition on the front vs. the back of the lens and the delivery of comfort enhancing components that could help to stabilize the tear film and enhance wettability. Although an important area of development, no myopia-control designs are available in SiHy materials, but researchers expect this to change. They also anticipate innovation for presbyopia with the addition of novel optical designs for multifocal contact lenses. With 20 years of lens advancements, the future looks promising for SiHy lenses. |
Innovation on the Horizon
Researchers from the Centre for Ocular Research & Education (CORE) at the School of Optometry & Vision Science at the University of Waterloo offer their insights on what’s ahead on the contact lens horizon. This research team is led by Lyndon Jones, PhD, DSc, FCOptom, CORE director, professor and university research chair, and includes Jill Woods, BSc (Hons), MCOptom, clinical research manager and senior clinical scientist at CORE; and Karen Walsh, BSc (Hons), MCOptom, CORE clinical scientist.
Myopia. They predict an expansion of myopia control designs, including the MiSight (CooperVision) design they have been working with in clinical trials. This soft lens for myopia control has been available in Canada for a year and even longer in some East Asian and European markets. “It will be interesting to see the impact of this lens in the US market once it gains the necessary FDA approvals to launch,” the CORE team says.
Another new soft myopia control lens that could hit the US market in the future is NaturalVue (Visioneering Technologies), which was available in some global markets in 2018.
“Given the worldwide recognition of the myopia epidemic, and the real sight-threatening pathology associated with high myopia, the focus on myopia control is crucial. We are excited by the rate at which our collective knowledge grows in this area and the fact that optical designs really do seem to have an impact,” CORE says. “While it is true that there is much we don’t understand, this is a fast moving area of research, with new evidence being generated, and new contact lens and spectacle designs being tested and released with increasing regularity.”
In addition to these products, the group is aware of alternate myopia management approaches via the use of orthokeratology, pharmaceutical treatments and the future potential of combination therapies that may bring together contact lens optical designs and drug delivery.
Light moderation. Light management will be of interest in 2019, the CORE team says. In addition to the photochromic lens on the way from JJV, there is much interest around eyestrain from digital device use.
“This has led to modifications to the optical designs of some contact lenses, targeted to reduce digital eyestrain. It is also possible that the digital light management technology offered in certain spectacle designs may translate into the contact lens market,” they add.
Biosensing. A growth in specialty medical lenses is also expected. Sensimed’s Triggerfish, a contact lens designed to evaluate changes in intraocular pressure, is commercially available in Europe and has FDA clearance.
“Biosensing technologies are a focus for many manufacturers and research institutions,” the CORE researchers say. “Parties are actively exploring the possibilities of this technology in a contact lens, as well as with other ocular and systemic applications, including detection of cancer markers, blood pressure monitoring, measuring tear film osmolarity and markers of dry eye disease.” While biosensing contact lenses are an exciting possibility, most technologies are still quite a few years away from being commercially available, the CORE team adds. Developers have several hurdles to overcome before bringing anything to market. In November 2018, Verily (Alphabet) and Alcon announced that they shelved development of a diabetes-monitoring contact lens, citing difficulties with obtaining consistent measures of glucose levels in tears.
Drug delivery. The CORE researchers also see growth in drug-delivering contact lenses to treat specific conditions, including glaucoma, inflammation, allergy and to aid ocular surface healing.
One such product in the pipeline is a JJV contact lens that includes the medication ketotifen fumarate. This drug-eluting contact lens is designed to help patients with itchy eyes due to ocular allergies, according to the company. JJV says it is on track to bring the product to market within five years.
 |
| Fig. 3. Patients with astigmatism now have a monthly replacement silicone hydrogel lens option in the form of Bausch + Lomb’s new Ultra Multifocal for Astigmatism, which is designed to provide stable, consistently clear vision and spherical aberration control in both axes to help reduce halos and glare. |
Contact Lens Wish Lists
Even though several new contact lenses are set to launch this year and others are working their way through the approval process, optometrists still hope additional advances will help to fulfill their patient needs.
“A daily disposable toric multifocal lens is next on my wish list,” Dr. Anderson says. “I have more than 65% of my patients wearing daily disposable lenses. The next area needed is the daily toric multifocal lens.”
There are still two key areas where patient needs could be better met, the CORE team says. “First are the rates of infection and inflammatory events.” Daily disposables are the best way to minimize risk of these adverse events, they say, but reusable contact lenses could benefit from the addition of antimicrobial properties to help reduce the incidence of these complications. “Secondly, we are striving to minimize dryness/discomfort, which remains the main reason for ceasing contact lens wear. New technologies that can enhance the comfort of the contact lens, such as release of tear-film type components when worn, would be welcome,” the CORE team says.
One example is the Tangible Hydra-PEG coating (Tangible Science), which recently gained FDA approval for use on daily disposable silicone hydrogel lenses. This coating is designed to improve wettability, increase surface water retention and lubricity, and minimize lens deposits. Tangible Science had previously licensed its technology for use with Bausch + Lomb’s rigid gas permeable and scleral contact lenses.
“We have a standing order with our gas permeable lenses right now to put Tangible on everything,” Dr. Brujic says. “That’s how good it’s actually been.”
Additionally, while research continues in the area of myopia control, new lens options for this patient population would be welcome sooner rather than later, doctors say. “Myopia management is a hot topic in optometry,” Dr. Bazan says. “I would like to see more contact lens companies develop options to help with the myopia epidemic.”
While everyone waits for their contact lens dreams to come true, ODs are looking forward trying several new lenses this year and sharing them with patients.
1. Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear: a meta-analysis. Optom Vis Sci. 2007;84(4):247-56. 2. Radford CF, Minassian D, Dart JK, et al. Risk factors for nonulcerative contact lens complications in an ophthalmic accident and emergency department: a case-control study. Ophthalmology. 2009;116(3):385-92. 3. Chalmers RL, Wagner H, Mitchell GL, et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study. Invest Ophthalmol Vis Sci. 2011;52(9):6690-6. 4. Chalmers RL, Keay L, McNally J, Kern J. Multicenter case-control study of the role of lens materials and care products on the development of corneal infiltrates. Optom Vis Sci. 2012;89(3):316-25. 5. Morgan PB, Woods C, Tranoudis I, et al. International contact lens prescribing in 2017. CL Spectrum. 2018;33(January):28-33. |