In 1836, without the aid of modern anesthesia, antiseptic techniques or surgical equipment, Dr. Richard Kissam attempted the first documented corneal transplant on a human, using a pig cornea and two sutures. From these humble beginnings, the field of modern corneal transplantation grew. While keratoplasty has advanced dramatically since that point—benefiting from breakthroughs in eye banking, surgical technique and postoperative immune suppression—the actual surgical procedure performed remained unchanged for more than 150 years following Kissam’s attempt.
Penetrating keratoplasty (PK) wore the crown for a century and a half. In the last 20 years, however, new methods of corneal transplantation have reignited interest in the procedure and radically changed the outcomes patients can expect. Work by Gerrit Melles, Mark Terry, Francis Price, Eduardo Arenas Archilla and Mohammed Anwar (among others) has led to the creation of the current lamellar surgical options known by their acronyms DSAEK, DMEK and DALK. These surgeries target only diseased tissue while leaving healthy cornea intact, creating tremendous potential benefits for the patient.
This article will outline the differences between the various surgeries, and their strengths and weaknesses relative to each other.
Penetrating Keratoplasty
Although PK is no longer the sole surgical option in the vast majority of transplants, it is still used in 15% of grafts, so maintaining some awareness of the procedure is important. Further, as the entire central cornea is replaced in this procedure, understanding the benefits and limitations of PK enhances our understanding of lamellar grafts and their strengths and weaknesses.
In PK, the entire corneal thickness is replaced over a 7.5mm to 8.0mm central zone and sutured into place. This procedure is known to have some benefits relative to lamellar surgeries: it’s easier to perform, allows for one surgery to be applied across all central corneal pathology and apparently—despite its limitations—may have a better average survival rate than the lamellar alternatives.1 However, these points are where its value ends.
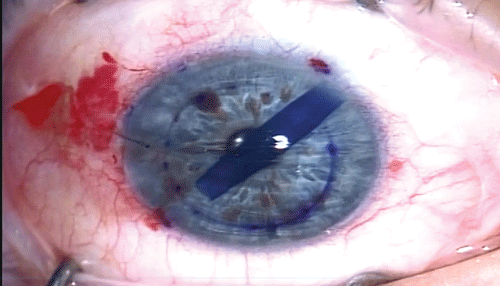 | |
| Fig 1. In this DMEK procedure, a scroll of donor DM tissue within the anterior chamber is stained with trypan blue for visualization. |
When compared to lamellar grafts, PK has three fatal flaws: first, as the most antigenic graft, PK is the transplant technique most likely to lead to a rejection episode and also the most likely to have that rejection episode culminate in failure of the graft; second, due to the extensive suturing needed to secure the graft, PK patients have visual recovery that is prolonged and characterized by time-consuming attempts to mitigate high levels of toricity, which can and often does drag out for years; and third, despite best efforts, refractive outcomes are often characterized by high levels of corneal irregularity, often leading to a high dependence on rigid gas permeable contact lenses.
While other real differences persist between PK and the lamellar surgeries, these three issues are what have led to the widespread embracing of lamellar techniques over PK.
Descemet’s Double Advantage
Descemet’s stripping automated endothelial keratoplasty (DSAEK) and Descemet’s membrane endothelial keratoplasty (DMEK) are the most recent steps on the surgical continuum that began in the late 1990s with Dr. Melles’ posterior lamellar keratoplasty (PLK), a treatment for corneal endothelial dysfunction such as Fuch’s dystrophy and pseudophakic bullous keratopathy (PBK). Taken as a group, endothelial decompensation historically accounted for nearly 50% of all indications for corneal transplant, making it the most frequently encountered indication for transplant overall and producing a true need for an improved surgery with quicker recovery and less dependence on GP lenses—a niche posterior lamellar transplants have filled.5
Both DSAEK and DMEK begin with a limbal or corneal incision, through which the host Descemet’s membrane (DM) and endothelium are removed over the central 8mm to 9mm or cornea. A folded or rolled graft (whose exact make-up and behavior is what distinguishes DSAEK from DMEK) is then placed through the same small limbal incision and unfolded within the anterior chamber. Once in position, the graft is then tamponaded with air or high-density gas, depending on the surgeon and the specific surgery performed.
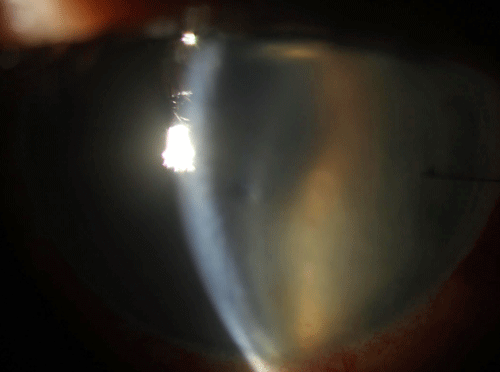
|
|
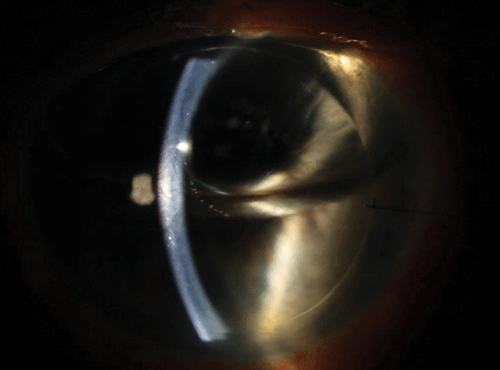
|
|
| Fig 2. Top photo: a DSAEK patient with a dislocation of the graft at day one. This is not a rejection episode. Note how edemetous the cornea is, and the graft floating in the AC. Bottom photo: one day after refloating the graft with a larger air bubble, it is in good position and the cornea is already remarkably clear. In cases where repositioning does not work, the graft is said to have failed and a repeat surgery is needed. |
Following the surgery, the patient is observed for a period prior to being sent home with some degree of air still within the anterior chamber, and positioning restrictions are given, with supine posture being recommended over the first few days to a week. This allows residual intracameral air to push the graft into place. Because of the air tamponade, combined with the natural suctioning effect of the endothelial pump, the donor graft will adhere to the host stroma in most cases, allowing for an effective transplantation of endothelium without the use of sutures.
Ignoring the differences between the exact make-up of DSAEK and DMEK grafts for a moment, it can be said that posterior lamellar grafts have some clear benefits over PK. As stated, much of the refractive challenge that comes with a PK owes to the creation of irregular corneal astigmatism by placement of sutures. No matter how skillfully placed, these sutures do not distribute tension evenly across the cornea and can result in marked irregularity of corneal curvature.
Posterior grafts avoid this in two ways: first, no sutures are used to secure the graft, helping to minimize corneal irregularity; second, even if the graft isn’t completely uniform in its curvature, it affects the posterior corneal curvature, which lessens the effect on overall refraction compared to changes to the anterior curvature as created with PK. The avoidance of irregular astigmatism is particularly valuable within the typical patient population needing keratoplasty for endothelial decompensation. Though no post-transplant patient would choose to depend on GP lenses for vision, such lenses represent a true complication in this generally elderly group where loss of dexterity may be a significant consideration.
The last benefit the lack of sutures generates comes in the form of visual recovery. In PK patients, refraction is not fully stable for several months after suture removal, a step that is often not undertaken until two years after surgery. This leads to final visual stability occuring somewhere around 30 to 40 months postoperatively. In an elderly patient population, waiting up to four years for vision to stabilize is perhaps not the best experience to improve that patient’s remaining years of life.
These concepts—GP lens use as a complication and prolonged visual recovery as a waste of a patient’s years of quality life—have greatly influenced the perception of and indications for PK compared to posterior transplants for the exact same indication. Previously, when a patient only had PK as an option for visual improvement, doctor and patient would wait until the pathology was causing significant visual debilitation, as the treatment results were often no better than mid-stage disease. In the current era of lamellar transplants, where the twin specters of GP lens use and prolonged visual recovery are no longer issues, doctors are intervening earlier in the disease process to provide patients with more high quality years of life.
|
Why’s the Endothelium So Important in Corneal Grafting?
First, of all tissue transplanted, only nucleated cells carry the necessary antigens to induce a rejection episode by the host immune system. Of the transplanted nucleated cells, only endothelial cells are non-miotic, and as such any damage they incur is permanently resident in the cornea; correspondingly, once they are transplanted, they persist indefinitely as a source of graft antigen. Transplanted epithelium and keratocytes, on the other hand, are replaced by host cells (as their stem cell regions fall outside the margins of the graft) at some point within the postoperative period—this occurs rapidly with epithelium, and slowly for keratocytes. Therefore, donor-derived corneal endothelium is the only possible permanent target of rejection. Second, once again owing to their non-miotic status, damage done to donor endothelium by an immunologic attack—when sufficient—can result in failure of the graft due to endothelial decompensation, making endothelial rejection the only type of rejection that can typically generate graft failure. Lastly, transplanted endothelial cell density (ECD) is reduced dramatically as a result of surgery, to the point that all transplants containing endothelium have a 35% to 45% reduction in ECD within the first year postoperatively. This likely correlates with an abbreviated life expectancy for grafts that contain endothelium, a feature that has been shown with PK and will likely bear out with posterior lamellar grafts as well. |
The last area where all posterior grafts exhibit some superiority over PK is as antigenic reservoirs. Though both PK and the posterior grafts transplant the permanently persisting antigen of donor endothelium, posterior grafts have a tendency toward preserving the immune privilege of the cornea whereas the sutures, proximity to deep stromal vascular beds, tendency towards induction of both cornea angiogenesis and lymphangiogeneis and chronic contact lens use with PK creates a pro-inflammatory micro-environment that results in the gradual erosion and loss of corneal immune privilege. This results in a higher rate of rejection episodes overall as well as a higher rate of severe rejection, which leads to failure with PK. This higher risk of rejection then requires a lengthier course of immune suppression with topical corticosteroids, which carry their own host of problems.
As stated earlier, the exact make-up of the graft is what differentiates DSAEK from DMEK. DSAEK grafts are comprised of donor endothelium and DM but also include a portion of posterior stroma, whereas DMEK grafts are made of endothelium and DM only. The stromal component of DSAEK grafts allow for slightly simpler graft preparation as well as intraoperative handling.
It is for these reasons that DSAEK is still the most frequently performed posterior lamellar surgery. Though the posterior stromal component of the graft offers real advantages for the surgeon intraoperatively, it appears to have a negative impact on graft optics, as DMEK patients generally have a quicker recovery and higher visual ceiling compared to a DSAEK equivalent population. In Price’s 2009 prospective study on DMEK, patients experienced a three-month BSVA of 20/25, which was equivalent to a one to two Snellen line improvement over the vision achieved by DSAEK grafts at six months.6
Further, though both of these grafts contain the exact same recognized level of graft antigen—the endothelium—Price’s research indicates that DMEK has a significantly (in fact, paradoxically) lower rate of rejection (1%) than DSAEK (9%) does. These results, if they hold over time, are indicative of some previously unrecognized level of importance that the generally acellular posterior stroma plays in mediating risk of rejection.7 Despite this significant difference of graft rejection rates between the two posterior transplants, the rate of graft failure as a result of the rejection episode is significantly lower with DSAEK than with PK and is probably more in line with that of DMEK.8
Despite the many virtues of posterior grafts, it is important to recognize that they do have limitations. First and foremost is the increased surgical complexity of these grafts relative to PK. Of the two transplants types, DMEK grafts are more challenging to handle intraoperatively, with tight scrolling of the graft a feature that has kept it from supplanting DSAEK as the most frequently performed poster lamellar transplant.
A second drawback of these grafts is the significantly higher rate of early failure compared to PK. Often inappropriately described as “graft rejection,” early failure of a posterior lamellar graft has to do with an insufficient endothelial pump function to create the suctional force necessary to hold the graft in place, allowing it to fall into the anterior chamber (in a PK, it simply manifests as an edematous, non-clearing graft). Most cases of graft dislocation do not represent graft failure and are able to be reattached with injection of more intracameral air; however, grafts that do not adhere after an attempt at repositioning are generally described as having failed.
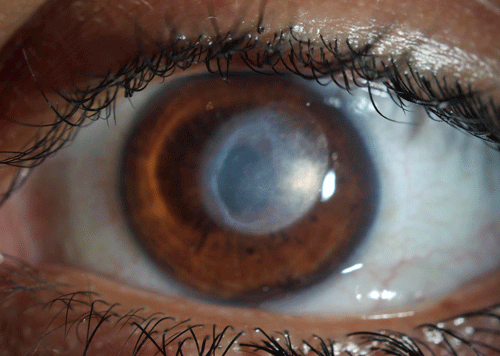
|
|
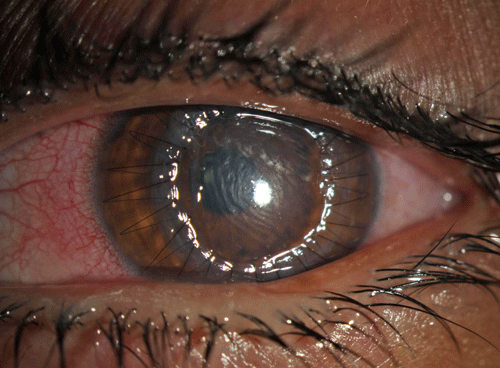
|
|
| Fig 3. This patient presented after treatment for a severe infectious ulcer (top photo). Because the resultant scar was too deep for PTK, DALK was elected. The bottom photo shows the typical appearance at one day post-op, with no epithelium present and mild stromal edema. Secured with a running suture, this is near-impossible to differentiate from a PK. |
DALK: The Opposite Approach
Despite being an acronym that looks similar to that of the posterior lamellar transplants, DALK is actually the exact opposite of a DMEK surgery. In deep anterior lamellar keratoplasty, all tissue anterior to DM and endothelium is transplanted and the host endothelium and DM are left behind. Even though only approximately 20µm—the thickness of DM and endothelium—separates a PK from a DALK, it has some important advantages over PK all having to do with its ability to maintain host endothelium.
Technically difficult and time-consuming, DALK requires manual dissection of host stroma paired with the injection of intracorneal air to pare down to the host DM. Due to potential for perforation of Descemet’s membrane, which occurs in as many as 32% of all attempted DALKs during this step, 18% all of DALK cases are converted to PK intraoperatively. Once the host stroma is removed, the donor tissue, which carries all tissue anterior to DM, is sutured into place. Because of the extensive suturing and clear optical interface at DM, in clinic it can be impossible to differentiate a DALK from a PK.
Even though DALK is a tedious surgery with high risk of intraoperative failure and patients experience the same slow visual recovery as PK (due to sutures), it has become the transplant of choice for all anterior pathology such as keratoectasias, deep scars and stromal dystrophies due to the immunologic benefits of maintaining the host endothelium.
Because the endothelium is not transplanted, a DALK graft has two dramatic advantages over a PK. First, non-transplanted endothelium loses cell density very gradually, whereas procedures that transplant the endothelium result in an accelerated loss of endothelium function. Because of this, DALK grafts are expected to persevere indefinitely without regrafting—a dramatic improvement over PK grafts, which have a finite uncomplicated lifespan of roughly 20 years. Within the patient population of keratoectasias, patients who need a regraft every 20 years may need three transplants in their lifetime. Second, there is a dramatic reduction in the risk of graft rejection with a near-absent risk of failure as a result of rejection. Ancillary benefits are then achieved by a lessened dependence on corticosteroids, which produces lower rates of steroid-induced glaucoma (a common source of vision loss with PKs), infection and cataract development.9
After well over a century of penetrating keratoplasty, the last two decades have moved eye care out of the era of full-thickness keratoplasty and into the era of lamellar keratoplasty. Never before have patients and doctors had the option to select a surgery that will treat only diseased tissue and spare healthy structures, resulting in a quicker recovery with less potential for complication. Understanding the options and their limitations will allow us to help patients select the most appropriate surgery for their pathology, as well as follow up with them appropriately.
Dr. Bronner is a staff optometrist at the Pacific Cataract and Laser Institute of Kennewick, Washington. He has no financial interest in any products or services described in this article.
1. Coster et al. A Comparison of Lamellar and Penetrating Keratoplasty Outcomes: A Registry Study. Ophthalmology. 2014;121: 979-987
2. Chen ES, Shamie N, Terry MA. Endothelial Keratoplasty: first case report of a ruptured globe after deep lamellar endothelial keratoplasty. Cornea, 2007; 26:874-5
3. Dursun D, Forster RK, Feuer WJ. Surgical technique for control of postkeratoplasty myopia, astigmatism and anistometropia. Am J Ophthalmology. 2003; 135: 807-15.
4. Terry MA, Ousley PJ. Deep lamellar endothelial keratoplasty visual acuity, astigmatism and endothelial survival in a large prospective series. Ophthalmology. 2005;112:1541-8
5. Maeno A, Naor J, Lee HM, Hunter WS and Rootman DS. Three decades of corneal transplantation: indications and patient characteristics. Cornea. 2000;19:7-11.
6. Price MO, Geibel AW, Fairchild KM and Price FW. Decemet’s Membrane Endothelial Keratoplast:Prospective Multicenter Study of Visual and Refractive Outcomes and Endothelial Survival. Ophthalmology. 2009;116:2361-2368
7. Anshu A, Price MO, Price FW. Risk of Corneal Transplant Rejection Significantly Reduced with Descemet’s Membrane Endothelial Keratoplasty. Ophthalmology. 2012; 119:536-540.
8. Ezon I et al. Immunologic Graft Rejection in Descemet’s Stripping Endothelial Keratoplasty and Penetrating Keratoplasty for Endothelial Disease. Ophthalmology. 2013; 120: 1360-5.
9. Ayyala R S. Penetrating Keratoplasty and Glaucoma. Survey of Ophthalmology. 2000; 43: 91-105
10. Cheng YY, et al. Endothelial Cell Loss and Visual Outcome of Deep Anterior Lamellar Keratoplasty vs Penetrating Keratoplasty: A Randomized Multicenter Clinical Trial. Ophthalmology 2011;118:302-309
11. Reinhart WJ. Et al. Deep Anterior Lamellar Keratoplasty as an Alternative to Penetrating Keratoplasty. A report by the American Academy of Ophthalmology. Ophthalmology. 2011; 118: 209-218


