The tear film is crucial for maintaining a healthy and comfortable ocular surface. When contact lens wearers experience dryness and discomfort, it is often from lens use. Experiencing recurrent contact lens discomfort leads to a reduction in both the number of hours, and eventually days, of wear for patients, to the point of dropping out of contact lens wear entirely.1 This article reviews the components of the tear film, its overall functions, the interactions that occur during lens wear and the actions most relevant for practitioners.
Composition and Function
The tear film is an extraordinarily complex, exquisite fluid, with many different components working together to deliver several important functions of vision, health and comfort related to the anterior eye. The appropriate balance of these components is crucial, as loss of homeostasis contributes to dry eye disease.2
The tear film over the ocular surface has a volume of approximately 7µL, a thickness of approximately 3µm to 5µm and is a highly complex biological fluid that comprises of over 1,500 unique proteins, more than 600 individual lipid species from 17 distinct lipid classes and up to 20 distinct mucin genes classified into two different types.3-9 Additional constituents include multiple small molecule metabolites, peptides, antioxidants, electrolytes and inflammatory mediators. The homeostasis of these components is crucial to the maintenance of several vital functions of the ocular surface, including hydration, lubrication, nutrition, protection and modulation of optical properties.
The initially proposed three-layer tear film structure—mucin, aqueous and lipid (Figure 1)—has been superseded by more contemporary theories of a multiple blended-phase tear film. Accordingly, a superficial lipid layer adjacent to an aqueous-mucin gel with an anchoring glycocalyx layer has been suggested to best describe the structure of the tear film over the ocular surface (Figure 2).10
The common proteins found in the tear film include lysozyme, lactoferrin, secretory immunoglobulin A (IgA) and lipocalin.7 Of these, lysozyme is the most abundant in tears and is capable of killing bacteria by breaking their outer cell walls.11 Lactoferrin provides antimicrobial efficacy in tears by binding free iron to reduce the availability of iron necessary for microbial growth and survival.12 Lactoferrin also plays an important role against inflammation by directly interacting with antigen-presenting cells, such as monocytes or macrophages, and modulating cytokine production.13 An essential component of the immune system, secretory IgA prevents the adhesion of microorganisms to the ocular surface by stimulating their ingestion.14,15 Lipocalin is a predominant lipid carrier in human tears and, due to its lipid binding properties, may integrate into meibomian lipids, leading to improved tear film stability and retardation of evaporation.16,17
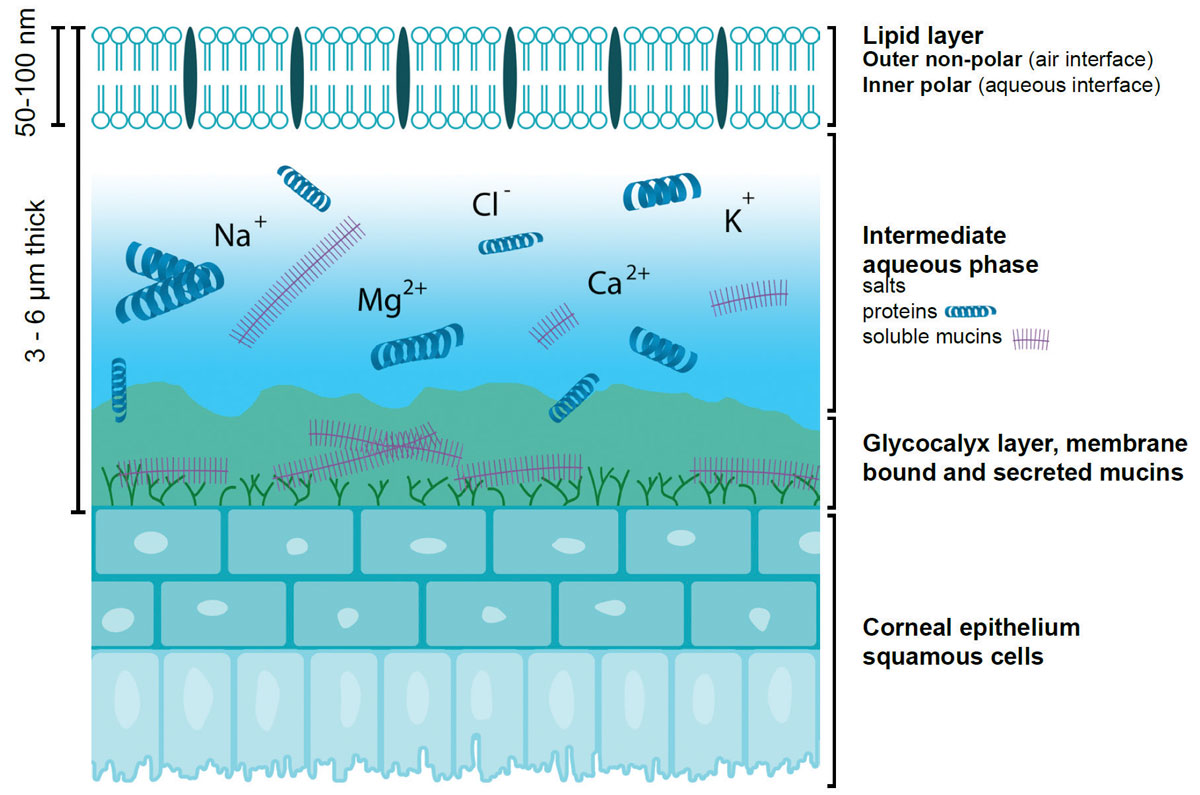 |
| Fig. 1. Graphic representation of the tear film. Adapted from Butovich IA, Millar TJ, Ham BM. Curr Eye Res. 2008;33(5):405-20. Click image to enlarge. |
Lipids in tears are broadly classified as polar and non-polar lipids. The non-polar lipids consist of fatty acids, cholesterol esters, diesters, free sterols, triglycerides and hydrocarbons, while the polar lipids primarily consist of phospholipids and omega hydroxy fatty acids.18 Many believe the non-polar lipids prevent tear evaporation, provide a clear optical surface and present an external barrier against foreign bodies.19,20
In contrast, polar tear lipids, via their amphiphilic properties, provide an intermediary between the outer non-polar lipid layer and inner aqueous layers of the tear film. This structure creates stability by lowering surface tension and increasing the viscoelasticity of aqueous tears. This promotes proper segregation of the tear film molecules, enables normal spreading of the tears and prevents dehydration of the ocular surface.18,21,22
Most of the mucins found in the tear film belong to two different sub-classes: membrane-spanning mucins and secretory gel-forming mucins. Membrane-spanning mucins are found close to the epithelial surface and play critical roles in protecting the cornea and conjunctiva by maintaining the hydration of the ocular surface and providing lubrication and anti-adhesive properties between the cells of the ocular surface and conjunctiva during blinking.23,24 Additionally, these mucins contribute to the epithelial barrier by restricting bacterial and viral access to the epithelium and participating in cell interactions.25 Furthermore, researchers hypothesize that gel-forming mucins are capable of trapping foreign bodies and pathogens and clearing them from the ocular surface into the nasolacrimal duct with the help of blinking.26
Although tear metabolites have not been studied extensively, about 60 small molecule metabolites have been identified, providing valuable insight into the dynamic biochemical processes occurring within the tear film.27 Among the antioxidants present in the tears, uric acid and ascorbic acid account for about 50%, with other examples including glutathione, cysteine, tyrosine and vitamin D.28,29 Antioxidants in tears help scavenge reactive oxygen species, which occur from UV exposure, radiation and pollutants, thereby protecting the eye from oxidative damage.30
More than over 200 different peptides have been detected in tears. These peptides, along with the inhibition of proteases and peptidases, are involved in antimicrobial response that may have potential therapeutic applications for some ocular diseases.31,32 Some electrolytes found in tears include sodium, potassium, calcium, magnesium, chloride and bicarbonate. Measurement of tear electrolytes may help identify and differentiate dry eye severity.33 Several other tear components that require further investigation. These include inflammatory mediators, such as IL-1 beta, increased concentrations of which have been reported with contact lens wear.34
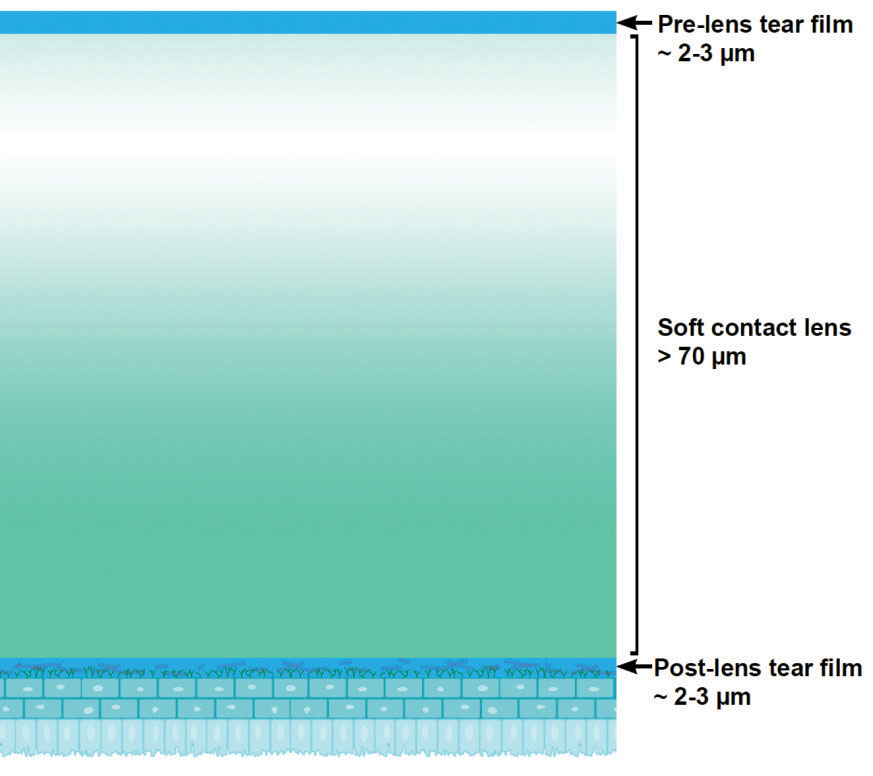 |
| Fig. 2. Graphic representation of a contact lens sitting with the pre- and post-lens tear film. Adapted from Butovich IA, Millar TJ, Ham BM. Curr Eye Res. 2008;33(5):405-20. |
Contact Lens Interactions
Because the tear film consists primarily of water and contains different components, it is not surprising that the concentrations of some of these components may be impacted by the presence of a contact lens (Table 1). Contact lenses interact with the tear film as soon as the two are exposed to each other.
When the lens is applied to the eye, the additional fluid from either the packaging solution or the care regimen dilutes the tears initially. Once in position, the contact lens splits the tear film into two distinct layers called the pre-lens and post-lens tear film.35 At the same time, tear film components start to deposit on the lens surface, with proteins being detectable on soft lens materials within seconds.36,37
In a healthy eye, tear film thickness is about 7µm; however, once a contact lens (which has a typical center thickness of at least 70µm) has settled on the eye, the pre-lens tear film is only about 1µm to 2µm.35 The tear film over the front surface of a soft lens has a thinner lipid layer, increased evaporation rate and reduced tear volume compared with the normal tear film.38,39 These changes destabilize the tear film, which may result in patients reporting dry eye symptoms during lens wear.40
Possibly related to increased tear evaporation, another change reported in contact lens wear is an increase in osmolarity from about 284mOsmol prior to lens wear to about 313mOsmol after three months of hydrogel lens wear.41 When soft contacts are worn, the noninvasive tear break-up time (NTBUT) typically reduces from 15 to 30 seconds prior to insertion to fewer than 10 seconds, irrespective of the material or wear regimen.42,43 NTBUT has been reported to be significantly different between “tolerant” (20 seconds) and “intolerant” (13 seconds) lens wearers when they are segregated based on their ability to tolerate lens wear for at least six hours.44
Whether the baseline protein, lipid or mucin profile of an individual’s tear film can be indicative of their potential for successful contact lens wear requires further investigation. However, research shows that the tear protein profile in lapsed lens wearers is different compared with that in non-lens wearers.45 One study found that the amount of total protein, lysozyme and lactoferrin were lower in previous lens wearers, while albumin levels and IgA-heavy chain were significantly higher, indicating the presence of enhanced immune activity weeks after lens wear was discontinued.45 The authors reported that ocular surface changes in intolerant contact lens wearers did not recover after a discontinuation period of three months, causing the recurrence of ocular discomfort symptoms during the refit attempt.45
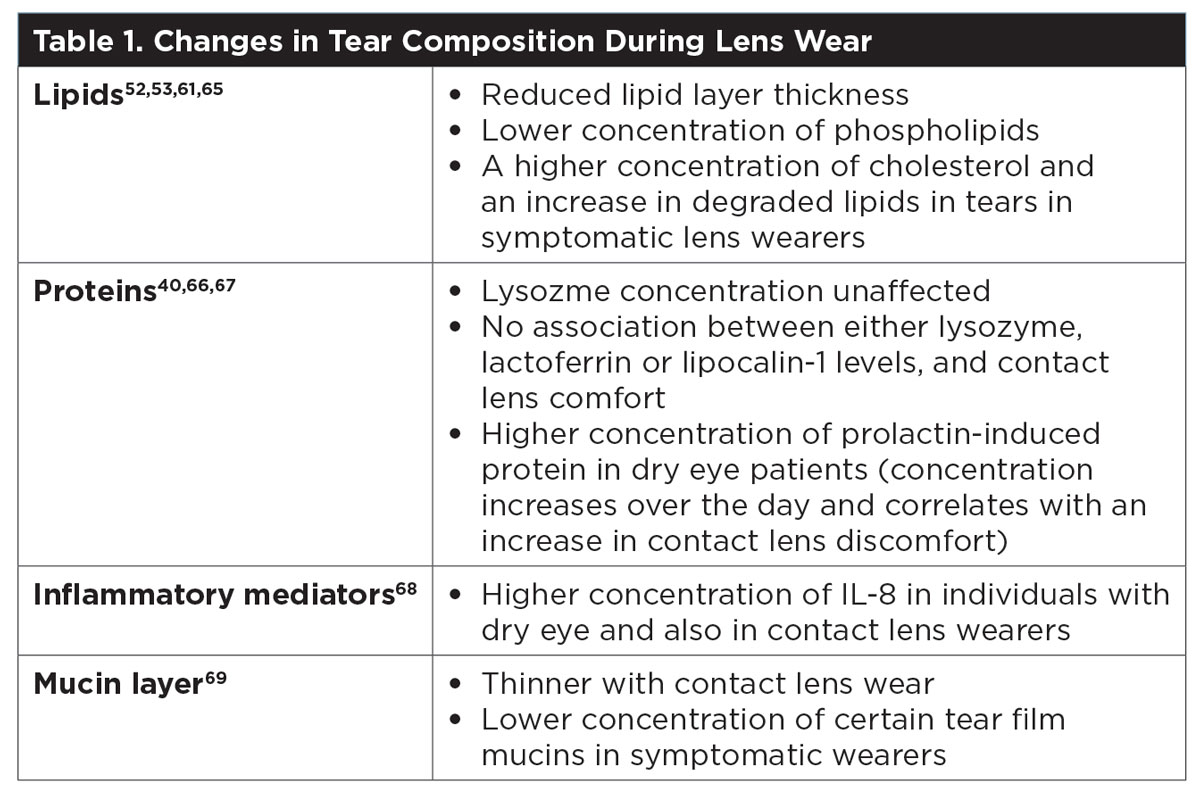 |
| Click to enlarge. |
The amount of protein and lipid that deposits on contact lenses is dependent on the material composition and other factors, such as overall charge, hydrophobicity and lens wear duration.36,46 Significant differences are seen between hydrogel and silicone hydrogel materials; however, few studies have found a link between tear film deposition and contact lens comfort or discomfort.47 It is desirable that deposited lysozyme remains in its active state to keep its antimicrobial properties, but studies often report a significant loss of activity once lysozyme binds to certain contact lens materials, which could impact comfort.48,49
The level of bacterial adhesion to contact lenses is increased in the presence of some proteins, although research shows that viable counts were reduced in the presence of lactoferrin deposits.50 If the ocular defense mechanism is compromised, the chance of developing an ocular inflammation or infection may increase.
A focus of current, ongoing contact lens material development is to optimize the way materials can work with the tear film rather than against it—integrating with the tear film rather than trying to resist its deposition.51 Further, new work is examining different surface modifications using components such as silver or melimine to resist the attachment of microorganisms to the lens surface and inhibit its activity. The ultimate goal is to produce contact lens materials that can maintain a healthy ocular surface and tear film homeostasis, keeping deposited components in their natural state while optimizing comfort for the wearer.
Clinical Application
The application of a contact lens onto the ocular surface splits the tear film in two, disrupting its structure and stability and altering the composition and concentration of some of its components.35, 40,42,43,52,53 Given this knowledge, what are the most relevant considerations and clinical findings for the eye care professional to keep in mind when reviewing their contact lens patients?
Clinicians should include a thorough assessment of the tear film in every examination of a contact lens wearer. While we currently lack evidence to be able to use the results of that assessment to inform choice of contact lens material or replacement frequency, evidence suggests that a combination of measures can indicate the likelihood a new patient will become a successful wearer.54-56 The combined results of baseline ocular symptoms, tear stability and tear volume can indicate whether the patient will be able to wear contact lenses comfortably.44,57,58
A simple approach for symptom assessment is asking the patient to describe how their eyes feel. Three or more descriptors such as dry or stinging eyes indicate an increased chance of being intolerant with contact lens wear.44 Quantify baseline symptoms by using a questionnaire, such as the Ocular Surface and Disease Index (OSDI), with the advantage of being able to monitor any change in scores over time.
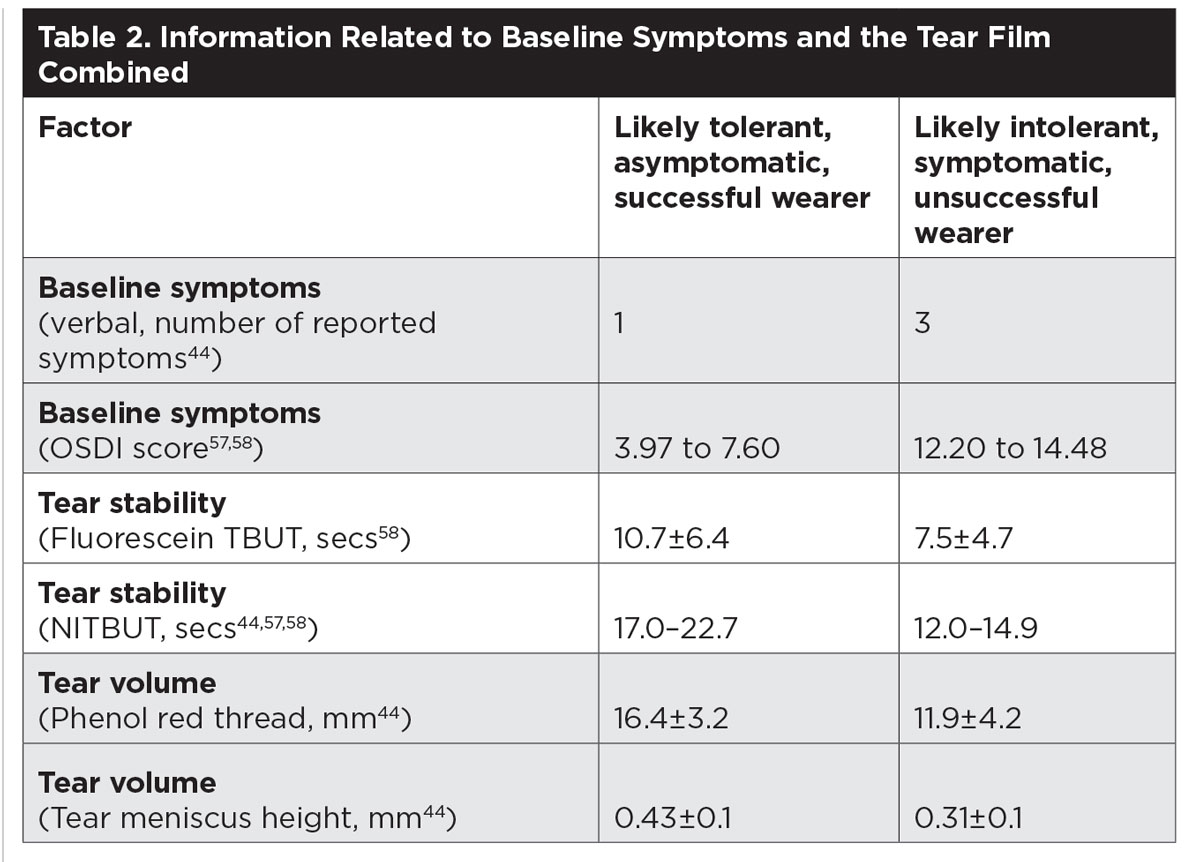 |
| Click to enlarge. |
While fluorescein break-up time has traditionally been and still is routinely used to assess tear stability, it is an invasive technique, with the instilled drop of fluorescein being around two to four times larger than the volume of the tear film it is trying to assess.59 The ability to use TBUT to distinguish asymptomatic and symptomatic wearers is improved when a practitioner uses a non-invasive technique. A Placido disc, keratometer mires or corneal topographer all enable NTBUT. Average NTBUT measures ranging between 12 to 15 seconds indicate the potential for less successful contact lens wear compared with successful or asymptomatic wearers who have an average NTBUTs ranging between 17 and 23 seconds.44,57,58
Tear volume can be estimated by invasive techniques such as the Schirmer test or phenol red thread test. These have particular application for the assessment of dry eye disease. Recording tear volume is also beneficial in contact lens wearers. It can be estimated through measuring the tear meniscus height along the lower lid margin. While this value is not particularly helpful in isolation, when combined with NTBUT and baseline ocular symptoms, it becomes a useful predictor of future contact lens intolerance (Table 2).44
The lipid layer of the tear film plays a crucial role in reducing tear evaporation. A severely compromised or absent lipid layer leads to evaporative dry eye.60 As contact lens wear disrupts the lipid layer, assessment of this particular tear film component is imperative.40,61 Even in the absence of having specialized equipment designed to estimate lipid layer thickness, check tear film lipids by paying close attention to the eyelids—specifically the lid margins and, the producers of the majority of tear film lipids, the meibomian glands.62 All contact lens wearers should have their meibomian glands examined. Check if the gland orifices are open or blocked. Can meibum be expressed, and what is the consistency of the meibum that is released? This information helps to build a image of how well these glands are functioning. Wherever suboptimal performance is found, appropriate management is necessary, including hot compresses, lid massage and hygiene, or in-practice treatments of lid debridement, exfoliation, heat and massage therapy.63,64
The tear film is incredibly complex, with a number of crucial roles in maintaining ocular health, comfort and vision. Components of the tear film interact with contact lens materials as soon as they come into contact, and the addition of a contact lens inevitably disrupts tears, resulting in changes at a molecular level and to its overall physical properties. Disruption of tear film homeostasis in contact lens wearers can lead to reduced comfort and wearing times, which may ultimately result in drop out from lens wear.
When reviewing contact lens wearers, it is worth paying close attention to the quality and quantity of the tear film, with specific focus on the function of the meibomian glands. For some clinical presentations, it can be helpful to introduce appropriate management as soon as possible to help improve tear quality. For new wearers, collect the results of symptoms and tear film measures together. These preliminary findings can help inform a useful discussion with the patient about their expectations related to the comfort and wearing hours they may be able to achieve with their lenses.
Ms. Walsh is a clinical scientist at the Centre for Ocular Research & Education at the University of Waterloo in Canada and is a Fellow of the International Association of Contact Lens Educators.
Dr. Dantam is a laboratory scientist at the Centre for Ocular Research & Education and is a Fellow of the American Academy of Optometry.
Dr. Luensmann is a clinical scientist at the Centre for Ocular Research & Education and is a Fellow of the American Academy of Optometry.
| 1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-83. 2. Nichols KK, Redfern RL, Jacob JT, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS14-19. 3. Mishima S, Gasset A, Klyce SD, Jr., Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol Vis Sci. 1966;5(3):264-76. 4. Wang JH, Fonn D, Simpson TL, Jones L. Precorneal and pre- and postlens tear film thickness measured indirectly with optical coherence tomography. Invest Ophthalmol Vis Sci. 2003;44(6):2524-28. 5. King-Smith PE, Fink BA, Hill RM, et al. The thickness of the tear film. Curr Eye Res. 2004;29(4-5):357-68. 6. King-Smith PE, Fink BA, Fogt N, et al. The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci. 2000;41(11):3348-59. 7. Zhou L, Zhao SZ, Koh SK, et al. In-depth analysis of the human tear proteome. J Proteomics. 2012;75(13):3877-85. 8. Lam SM, Tong L, Duan X, et al. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res. 2014;55(2):289-98. 9. Hodges RR, Dartt DA. Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp Eye Res. 2013;117:62-78. 10. Pflugfelder SC, Solomon A, Stern ME. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. 2000;19(5):644-9. 11. Fullard RJ, Snyder C. Protein levels in nonstimulated and stimulated tears of normal human subjects. Invest Ophthalmol Vis Sci. 1990;31(6):1119-26. 12. Flanagan JL, Willcox MD. Role of lactoferrin in the tear film. Biochimie. 2009;91(1):35-43. 13. Puddu P, Valenti P, Gessani S. Immunomodulatory effects of lactoferrin on antigen presenting cells. Biochimie. 2009;91(1):11-8. 14. Williams RC, Gibbons RJ. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972;177(4050):697-9. 15. Willcox MD, Lan J. Secretory immunoglobulin A in tears:functions and changes during contact lens wear. Clin Exp Optom. 1999;82(1):1-3. 16. Glasgow BJ, Gasymov OK. Focus on molecules: tear lipocalin. Exp Eye Res. 2011;92(4):242-3. 17. Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci. 2009;50(1):140-51. 18. McCulley JP, Shine W. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79-93. 19. Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci. 1997;74(1):8-13. 20. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922-9. 21. Rosenfeld L, Fuller GG. Consequences of interfacial viscoelasticity on thin film stability. Langmuir. 2012;28(40):14238-44. 22. Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res. 2003;26(2):89-94. 23. Argueso P. Glycobiology of the ocular surface: mucins and lectins. Jpn J Ophthalmol. 2013;57(2):150-5. 24. Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78(3):379-88. 25. Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Cl. 2008;8(5):477-83. 26. Gipson IK, Argüeso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol. 2003;231:1-49. 27. Chen LY, Zhou L, Chan ECY, et al. Characterization of the human tear metabolome by LC-MS/MS. J Proteome Res. 2011;10(10):4876-82. 28. Choy CKM, Benzie IFF, Cho P. Ascorbic acid concentration and total antioxidant activity of human tear fluid measured using the FRASC assay. Invest Ophthalmol Vis Sci. 2000;41(11):3293-8. 29. Gogia R, Richer SP, Rose RC. Tear fluid content of electrochemically active components including water soluble antioxidants. Curr Eye Res. 1998;17(3):257-63. 30. Chen Y, Mehta G, Vasiliou V. Antioxidant defenses in the ocular surface. Ocul Surf. 2009;7(4):176-85. 31. Azkargorta M, Soria J, Ojeda C, et al. Human basal tear peptidome characterization by CID, HCD, and ETD followed by in silico and in vitro analyses for antimicrobial peptide identification. J Proteome Res. 2015;14(6):2649-58. 32. Pescosolido N, Barbato A, Pascarella A, et al. Role of protease-inhibitors in ocular diseases. Molecules. 2014;19(12):20557-69. 33. Yetisen AK, Jiang N, Tamayol A, et al. Paper-based microfluidic system for tear electrolyte analysis. Lab Chip. 2017;17(6):1137-48. 34. Yüksel Elgin C, İskeleli G, Talaz S, Akyol S. Comparative analysis of tear film levels of inflammatory mediators in contact lens users. Curr Eye Res. 2016;41(4):441-7. 35. Nichols JJ, King-Smith PE. Thickness of the pre- and post-contact lens tear film measured in vivo by interferometry. Invest Ophthalmol Vis Sci. 2003;44(1):68-77. 36. Luensmann D, Jones L. Protein deposition on contact lenses: the past, the present, and the future. Cont Lens Anterior Eye. 2012;35(2):53-64. 37. Hall B, Jones L, Forrest JA. Measuring the kinetics and activity of adsorbed proteins: in vitro lysozyme deposited onto hydrogel contact lenses over short time periods. J Biomed Mater REs A. 2013;101(3):755-64. 38. Lloyd AW, Mahalingham N, Guillon M. Tear evaporation in contact lens wear. ARVO 2004. Invest Ophthalmol Vis Sci. 2004;45:3890. 39. Chen Q, Wang J, Shen M, et al. Tear menisci and ocular discomfort during daily contact lens wear in symptomatic wearers. Invest Ophthalmol Vis Sci. 2011;52(5):2175-80. 40. Craig JP, Willcox MD, Argueso P, et al. The TFOS international workshop on contact lens discomfort: report of the contact lens interactions with the tear film subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS123-56. 41. Iskeleli G, Karakoc Y, Aydin O, et al. Comparison of tear-film osmolarity in different types of contact lenses. CLAO J. 2002;28(4):174-6. 42. Keir N, Jones L. Wettability and silicone hydrogel lenses: a review. Eye Contact Lens. 2013;39(1):100-8. 43. Morris CA, Holden BA, Papas E, et al. The ocular surface, the tear film, and the wettability of contact lenses. Adv Exp Med Biol. 1998;438:717-22. 44. Glasson MJ, Stapleton F, Keay L, et al. Differences in clinical parameters and tear film of tolerant and intolerant contact lens wearers. Invest Ophthalmol Vis Sci. 2003;44(12):5116-24. 45. Giannaccare G, Blalock W, Fresina M, et al. Intolerant contact lens wearers exhibit ocular surface impairment despite three months wear discontinuation. Graefes Arch Clin Exp Ophthalmol. 2016;254(9):1825-31. 46. Jones L, Brennan NA, Gonzalez-Meijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS37-70. 47. Subbaraman LN, Omali NB, Heynen M, et al. Could lipid deposition on contact lenses be beneficial?. Presentation at: BCLA Clinical Conference and Exhibition. June, 2014; Birmingham, UK. 48. Jones L, Senchyna M, Glasier MA, et al. Lysozyme and lipid deposition on silicone hydrogel contact lens materials. Eye Contact Lens. 2003;29(1 Suppl):75-9. 49. Subbaraman LN, Glasier MA, Varikooty J, et al. Protein deposition and clinical symptoms in daily wear of etafilcon lenses. Optom Vis Sci. 2012;89(10):1450-9. 50. Subbaraman LN, Borazjani R, Zhu H, et al. Influence of protein deposition on bacterial adhesion to contact lenses. Optom Vis Sci. 2011;88(8):959-66. 51. Buch J, Canavan K, Fadli Z, Scales C. The tear film and contact lens wear. Contact Lens Spectrum. 2016;31(2):34-7. 52. Yamada M, Mochizuki H, Kawashima M, Hata S. Phospholipids and their degrading enzyme in the tears of soft contact lens wearers. Cornea. 2006;25(10 Suppl 1):S68-72. 53. Glasson M, Stapleton F, Willcox M. Lipid, lipase and lipocalin differences between tolerant and intolerant contact lens wearers. Curr Eye Res. 2002;25(4):227-35. 54. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-74. 55. Mousavi M, Jesus DA, Garaszczuk IK, et al. The utility of measuring tear film break-up time for prescribing contact lenses. Cont Lens Anterior Eye. 2018;41(1):105-9. 56. Ruiz-Alcocer J, Monsalvez-Romin D, Garcia-Lazaro S, et al. Impact of contact lens material and design on the ocular surface. Clin Exp Optom. 2018;101(2):188-92. 57. Pult H, Murphy PJ, Purslow C. A novel method to predict the dry eye symptoms in new contact lens wearers. Optom Vis Sci. 2009;86(9):E1042-50. 58. Best N, Drury L, Wolffsohn JS. Predicting success with silicone-hydrogel contact lenses in new wearers. Cont Lens Anterior Eye. 2013;36(5):232-7. 59. Mooi JK, Wang MTM, Lim J, et al. Minimizing instilled volume reduces the impact of fluorescein on clinical measurements of tear film stability. Contact Lens Anterior Eye. 2017;40(3):170-4. 60. Craig J, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vision Sci. 1997;74(1):8-13. 61. Young G, Efron N. Characteristics of the pre-lens tear film during hydrogel contact lens wear. Ophthalmic Physiol Opt. 1991;11(1):53-58. 62. Butovich IA. Tear film lipids. Exp Eye Research. 2013;117:4-27. 63. Arita R, Fukuoka S, Morishige N. Meibomian gland dysfunction and contact lens discomfort. Eye Contact Lens. 2017;43(1):17-22. 64. Geerling G, Tauber J, Baudouin C, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on management and treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52(4):2050-64. 65. Young WH, Hill RM. Tear cholesterol levels and contact lens adaptation. Am J Optom Arch Am Acad Optom. 1973;50(1):12-6. 66. Masoudi S, Stapleton FJ, Willcox MD. Contact lens-induced discomfort and protein changes in tears. Optom Vis Sci. 2016;93(8):955-62. 67. Zhou L, Beuerman RW, Chan CM, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8(11):4889-905. 68. Poyraz C, Irkec M, Mocan MC. Elevated tear interleukin-6 and interleukin-8 levels associated with silicone hydrogel and conventional hydrogel contact lens wear. Eye Contact Lens. 2012;38(3):146-9. 69. Berry M, Pult H, Purslow C, Murphy PJ. Mucins and ocular signs in symptomatic and asymptomatic contact lens wear. Optom Vis Sci. 2008;85(10):E930-8. |


