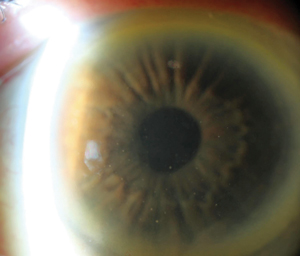
 | |
Diffuse keratitic precipitates are an atypical distribution except in cases of Fuchs’ heterochromic iridocyclitis and herpetic uveitis. This is a case of HSV uveitis. |
Herpetic eye disease is the number one cause of infectious vision loss in the United States.1 Though non-corneal manifestations of the disease exist, corneal ulceration, infiltration and scarring are the primary mechanisms through which this group of viruses cause vision loss. As such, viral eye disease is also the primary infectious indication for keratoplasty. While penetrating keratoplasty (PK) and deep anterior lamellar keratoplasty (DALK) are effective at re-establishing a clear cornea, eyes with herpetic indication for transplant have an elevated risk of viral reactivation within the donor tissue in the first year postoperatively. This perpetuates the cycle of infection, inflammation and scarring and may prompt a need for retransplant.2,3
In initial episodes, these sorts of severe outcomes may seem only remotely possible and foster a false sense of security when initial medical treatment is successful. However, herpes viruses are capable of remaining dormant within the host and reactivating at a later date; thus, treating these eyes effectively over both the acute and chronic phase of the disease are clinical challenges that must be resolved.
Compounding the therapeutic challenges posed by viral eye disease are some of the common points of confusion in its diagnosis and management—namely, antiviral efficacy, the role of steroids in management, the diagnosis of herpetic iridocyclitis, differences in medication response between herpes simplex and herpes zoster and the effect and role of vaccination in the epidemiology and management of herpes zoster ophthalmicus (HZO).
The purpose of this article is to review some of the more technical considerations regarding herpetic eye disease and how we can apply this to our patients to achieve successful long-term outcomes.
The Vagaries of Vaccines: Did Varivax Create a Shingles Boom? Notably, these relationships were disrupted by the widespread varicella vaccination process begun two decades ago when Varivax (Merck) was added to the recommended childhood vaccination schedule in 1995. This vaccine is made up of a live, albeit attenuated, form of VZV known as the Oka strain. The virus type is more easily contained by the immune system, which reduces disease burden. As such, clinical varicella has been reduced by approximately 70%.22,23 This reduced disease burden, however, may have some unintended consequences for anyone carrying wild-type VZV in latency—namely, all of us whose childhood immunization panels predate the introduction of Varivax. A widely held (though unproven) belief is that a significant part of our immune-boosting capabilities against the virus, which influences our immune vigilance, comes with environmental exposures to children who have varicella.23 The Varivax program thus deprives us of these immune boosters, as children are no longer widely developing chicken pox. If, as seems likely, highly transmissible varicella provides immune boosters for those with latent VZV, immune vigilance will wane more rapidly, and we could see a sharp rise in shingles among the pre-vaccine generations, possibly including healthy adults. This rise is predicted by mathematical modeling to occur 10 to 20 years after implementation of the vaccine—i.e., now—and could last for 30 to 50 years.24 At this time, efforts to document a potential increase in zoster incidence have been inconsistent.23 My own anecdotal experience has been marked by a spate of otherwise healthy young adults developing zoster. The long-term projection for shingles is a rosier picture. The Oka strain is more easily contained by the immune system, preventing initial manifestation of chicken pox, and more easily held in latency, which theoretically should reduce zoster incidence in adulthood among those vaccinated. Of course, this effect will not manifest until all pre-vaccination generations have died. Although we won’t be around to see it, one small part of the world we’ll leave our children could be better—less shingles. For us “great unwashed masses” of pre-vaccination, Zostavax (Merck), which uses the same Oka strain as Varivax but at significantly higher concentrations, offers protection against shingles as in immune booster. It has been shown to reduce incidence of zoster by 50% in susceptible populations. Approved in 2006, the CDC currently recommends Zostavax for all adults over the age of 60. It is approved for use in patients as young as 50. This gives rise to a common clinical question: “My patient just had shingles; should I send them for Zostavax?” The answer is probably “no.” The episode of shingles is likely to be at least as potent an immune booster as the vaccine, and so vaccination will provide diminishing returns, if any. However, patients who are at risk for shingles and in the absence of an immediate history of the disease should likely be encouraged to receive the booster. For high-risk, immune-suppressed populations with potential for recurrent disease, suppression dosing of antiviral is probably more appropriate than the vaccination. |
Simplex: Not so Simple
Herpes simplex 1 and 2 (HSV-1 and HSV-2) and varicella zoster (VZV) are members of the alphaherpesvirinae subfamily of the larger herpesviridae family, a group to which cytomegalovirus and Epstein Barr virus also belong. HSV is the primary source of viral-induced vision loss affecting approximately 500,000 patients in the United States, with 10% to 20% developing ocular effects.1,4,5 Though the HSV genome is moderately larger than VZV (due to coding repeats), there is a surprising genetic homology for viruses that can result in different pathologies: HSV-1 has only 10 genes that VZV does not, while VSV has only six genes that HSV-1 does not.6 In addition to their genetic resemblance, the viruses produce similar findings clinically (i.e., dendriform keratitis, disciform endotheliitis, iridocyclitis, retinitis and neurotrophy). As such, many practitioners treat these viruses in a similar fashion. There are several problems with this approach, however:
First, the Herpetic Eye Disease Study (HEDS), on which the majority of clinical practices regarding viral eye disease are based, looks only at herpes simplex, not varicella zoster—which limits the patent base to which data from the study can be applied to.
Second, despite the responsiveness of both HSV and VZV to guanosine antivirals, their natural histories, ocular effects and specific antiviral sensitivities can vary significantly. Herpes simplex and herpes zoster both begin as epithelial disease; however, once established as affecting the cornea, HSV may vacillate freely between epithelial recurrences of infection and immune stromal keratitis as well as iridocyclitis, and may have a tendency towards frequent recurrences. This is in contrast to zoster ophthalmicus, which progresses more typically on a temporal continuum, with some cases resolving early in the process and others progressing to chronic smoldering ocular inflammation. Zoster ophthalmicus also much less frequently involves multiple recurrences. Therefore, while the management of HSV keratitis involves episodic treatments of acute flare-ups, zoster keratitis, while less common, can sometimes take months to effectively clear and may be marked by periodic manifestations of different forms of the disease.
The differing antiviral sensitivity patterns among virus groups also pose problems. Zoster research lags significantly behind that of simplex due to the lack of animal models of the disease and difficulties with experimental reactivation in humans, challenges that HSV research doesn't face.5 The second-generation antiviral trifluridine, which is too toxic for systemic dosing, is effective topically in the management of infectious HSV, but is ineffective against VZV.3 Among the guanosine analog agents (acyclovir, valacyclovir, famciclovir and ganciclovir) one finding remains consistent: HSV has two to four times lower minimum inhibitory concentrations than varicella zoster virus, a fact reflected in the higher dosing strategies of oral antivirals for zoster compared with simplex.7-10 Of this group, ganciclovir, an effective agent that is toxic when dosed systemically, is the only member of this class commercially available as a topical preparation in the United States (i.e., ganciclovir 0.15% gel, Zirgan, Bausch + Lomb).7
Suppression Dosing of Oral Antivirals
One of the most important elements to come out of HEDS was the role of suppression dosing of oral antivirals, which significantly reduce the rate of recurrence of both stromal and epithelial keratitis with HSV infection. Dosing 400mg of acyclovir BID reduced the rate of recurrent stromal disease—the manifestation most likely to lead to corneal transplant among patients who had already had at least one episode of stromal keratitis—from 28% in the placebo group to 14% in the treatment group. Note, this protective effect unlikely extends after treatment ceases.12,13,14 Further, while years of research indicated that acyclovir-resistant HSV was extremely uncommon in immune competent patient bases, a more recent study concluded that long-term (i.e, greater than one year) suppression dosing was a significant risk factor for promoting acyclovir resistance, even in normal patients.15 Therefore, using suppression dosing is warranted only when a history of recurrent stromal disease supports it.
While this HEDS-based information can’t be applied to the treatment of HZO—a virus with a tendency towards much less recurrence among healthy populations—a handful of smaller studies have demonstrated suppression dosing is effective at reducing incidence of herpes zoster in immune-suppressed populations.14,15
I am comfortable extrapolating this information to my clinical care of immunocompetent chronic or recurrent HZO and use a suppression dose in cases where I feel it’s warranted. Keep in mind, however, in most cases the most effective means of boosting immunity to the virus is with an activation and subsequent immune response, so suppression dosing is not warranted in most uncomplicated, non-recurrent cases of HZO.
| HEDS Up: Three Key Points The first Herpetic Eye Disease Study (HEDS I) is over 20 years old and—though it is still being presented as such—is no longer new material. At this point, eye care providers must be aware of the following elements of the study: 1. It only applies to herpes simplex; varicella zoster was not studied. |
The Role of Steroids
The clinical manifestations of herpes simplex keratitis vary from dendritic epithelial disease to sub-dendritic stromal keratitis, nummular keratitis and disciform endotheliitis. The generally accepted mechanism of these keratitides is that dendritic disease is actually infectious keratitis characterized by proliferation of live virus within the epithelial margins of the lesion, while stromal and endothelial effects are inflammatory responses to retained non-vital viral proteins. As such, it’s useful to sub-classify HSV keratitis as infectious epithelial keratitis or immune stromal keratitis and treat as the underlying mechanism indicates. For infectious epithelial disease, an antiviral (either oral or topical) is sufficient.18 For immune stromal disease, topical steroids and a paired prophylactic topical or oral antiviral is appropriate treatment.
Clinically, my approach to HSV keratitis is to use a topical or oral antiviral in cases of simple dendritic keratitis. Research shows either approach to be equivalent.14 When the pathology moves deeper, though, I become more particular about treatment regimens. In cases of inflammatory sub-dendritic, nummular or disciform keratitis, I use a topical corticosteroid paired with an oral antiviral for prophylaxis, as effective penetration of the topically dosed antivirals becomes a concern. When manifestations transition from infectious to inflammatory, I treat the infectious process first and then add a steroid once the cornea has been sterilized, as indicated by re-epithelialization.
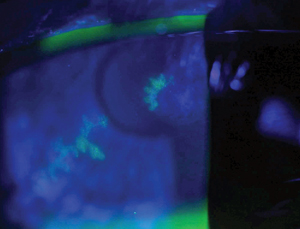 | |
| A presentation of classic dendritic keratitis due to HSV. |
Because topical steroids have the potential to make cases of HSV epithelial keratitis worse, their use for all manifestations of HSV keratitis inappropriately has remained somewhat taboo. The good news is we know that while topical corticosteroid use is important to outcomes, its precise timing is not, nor are the use of these agents for the treatment of HSV stromal keratitis likely to cause a recurrence of the infectious epithelial disease when paired with antiviral therapy.17,18 Therefore, if you are uncomfortable treating with a steroid immediately with the concern of reactivating the virus, delaying for a week or two to allow more effective clearance of the organism should not impact outcomes.17
The role of corticosteroids in managing herpes zoster ophthalmicus is a different animal. This distinction is illustrated by the differences in prescribing practices in the treatment of non-ocular disease of both HSV and VZV. An internist would not prescribe an oral steroid to treat severe HSV infection, as its use may perpetuate and worsen the underlying infection.
However, systemic corticosteroids are used in immune-competent patients during episodes of shingles to reduce associated pain, without concern for disease exacerbation. Mirroring this systemic practice is the use of topical steroids with HZO. Although there are widely accepted manifestations of the disease that are active viral proliferation—the pseudodendrite, for example—corticosteroids are not strictly contraindicated in any HZO presentation. Though widespread, use of corticosteroids in treatment of HZO is not universally accepted.13 Rather, some practitioners feel their use may prolong episodes and may predispose a patient to further, more severe manifestations of the disease.4 Because of this controversy, a general prescribing practice of corticosteroids in the management of HZO is to be comfortable using them but only when their absence threatens vision, as in the case of central or severe corneal infiltrates or recrudescent inflammation. Also, as HZO often progresses along many months, it’s important to realize that dosing of steroids may require a several month taper.
 | |
| Sectorial iris atrophy in a case of herpes zoster uveitis. |
Herpetic Uveitis
The clinical picture of herpetic uveitis can be quite variable, with fine or granulomatous keratic precipitates that may be distributed diffusely, in Arlt’s triangle or in a linear pattern. Concomitant corneal edema, IOP spikes or sectorial iris atrophy may also appear.21,22,23 While this variability implies there won’t be one classic presentation of viral uveitis, it also gives a number of clinical clues about potential diagnoses.
Equally valuable in arriving at a correct diagnosis for viral uveitis is the patient setting. In cases of anterior uveitis, the most common causative systemic etiologies are: idiopathic, HLA-B27 associated disease, sarcoidosis and herpetic disease. Of this group, new diagnoses decrease significantly in patients over age 60 for all causes except herpetic disease, as most cases of autoimmune disease develop and are established prior to age 60. Its expanding presence in this age group should not be a surprise—just as immune senescence increases likelihood of herpes zoster, ocular involvement should also be expected to increase as we age; in fact, uveitis may be the most common ocular manifestation of the disorder.
In a review of patients over the age of 60 who developed uveitis for the first time, herpes zoster and herpes simplex were the most common non-idiopathic etiologies found, accounting for approximately 18% of uveitis cases.20 An appropriate differential diagnosis for presumed viral uveitis in this age group would contain other common sources of uveitis, but perhaps more importantly, consideration of the masquerading syndromes of intraocular leukemia or lymphoma, as well as a retained cataract fragment after cataract surgery, should be given.
As penetration of topical antivirals into the anterior chamber can be hampered by an intact epithelium, treatment of isolated viral uveitis should include both oral antivirals at the correct treatment dosage for the suspected etiology, and a topical, and in some cases oral, corticosteroid.
The management of viral eye disease, due to its prolonged natural history, relationship with host immunity and varied clinical presentations, is a complex and serious part of eye care. Understanding these subtleties and recognizing the differences between HSV and VZV can improve practitioner confidence and patient care, and lead to better outcomes.
Dr. Bronner is a staff optometrist at the Pacific Cataract and Laser Institute of Kennewick, Wash. He has no financial interest in any products or services described in this article.
1. Farooq A, Sukla D. Herpes simplex epithelial and stromal keratitis: An epidemiologic update. Survey of Ophthalmology. 2012; 57: 448-62.
2. Al-Yousuf N, Mavrikakis I, Mavrikakis E, Daya SM. Penetrating keratoplasty: indications over a 10 year period. Ophthalmol. 2004; 88:998-1001.
3. Lomholt JA, Baggesen K, Ehlers N. Recurrence and rejection rates following corneal transplantation for herpes simplex keratitis. Acta Ophthalmol Scand. 1995;73:29-32.
4. Barry Lee W, Liesegang TJ. Herpes Zoster Keratitis. In: Krachmer JH, Mannis MJ, Holland EJ eds. Cornea. 2nd ed. St Louis: Mosby;2004:1075-90.
5. Borkar DS et al. Incidence of Herpes Zoster Ophthalmicus: Results from the Pacific Ocular Inflammation Study. Ophthalmol. 2013;120: 451-56.
6. Kinchington PR, St. Leger AJ, Guedon JM, Hendricks RL. Herpes simplex virus and varicella zoster virus, the house guests who never leave. Herpesviridae. 2012;3: 1-13.
7. Clercq ED, Field HJ. Antiviral prodrugs –the development of successful prodrug strategies for antiviral chemotherapy. British Journal of Pharmacology. 2006;147:1-11.
8. Biron K, Elion GB. In Vitro Suceptibility of Varicella-Zoster Virus to Acyclovir. Antimicrobial Agents and Chemotherapy. 1980;18:443-7.
9. Cobo et al. Oral Acyclovir in the Treatment of Acute Herpes Zoster Ophthalmicus. Ophthalmol. 1986;93:764-70.
10. Hoang-Xuan T, et al. Oral Acyclovir for Herpes Zoster Opthalmicus. Ophthalmol. 1992;99:1062-70.
11. Aggarwal S. et al. Treatment of Pseudodendrites in Herpes Zoster Ophthalmicus with Topical Ganciclovir 0.15% Gel. Cornea. 2014;109-113.
12. Wilhelmus K et al. Acyclovir for the Prevention of Recurrent Herpes Simplex Virus Eye Disease. The Herpetic Eye Disease Study Group. The New England Journal of Medicine. 1998; 339:300-6.
13. Sy A I et al. Practice Patterns and Opinions in the Management of Recurrent or Chronic Herpes Zoster Ophthalmicus. Cornea. 2012; 31: 786-90.
14. Cernik C, Gallina K, Brodell RT. The Treatment of Herpes Simplex Infections: An evidence based review. JAMA. 2008; 168:1137-44.
15. vanVelzen M, et al. Acyclovir prophylaxis predisposes to antiviral-resistant recurrent herpetic keratitis. J Infect Dis. 2013;9: 1359-65.
16. Seok JK, Kim K, Do YR, et al. Low-dose Acyclovir is Effective for Prevention of Herpes Zoster in Myeloma Patients treated with Bortezomib: A report from the Korean Multiple Myeloma Working Party (KMMWP) Retrospective Study. Japanese Journal of Clinical Oncology. 2011; 41: 353-7.
17. Ljungman P. Prophylaxis against herpesvirus infections in transplant recipients. Drugs. 2001;61:187–96.
18. Wilhelmus K. Therapeutic interventions for herpes simplex virus epithelial keratitis (Review). The Cochrane Collaboration; 2009;1-130.
19. Wilhelmus KR, Gee L, Hauck WW, et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmol. 1994; 101:1883-95.
20. Wilhelmus K, Dawson CR, Barron BA, et al. Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. Ophthalmol. 1996;80:969-72.
21. Jap A, Chee SP. Viral anterior uveitis. Current Opinion in Ophthalmology. 2011; 22:483-88.
22. Chatzistefanou K et al. Characteristics of Uveitis Presenting for the First Time in the Elderly. Ophthalmol. 1998; 105:347-52.
23. Van Gelder RN, Prasad AG. Review of Uveitis. 2008 Slack Publishing.
24. Holland EJ, Brilakis HS, Schwartz GS. Herpes Simplex Keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, eds. Cornea. 2nd ed. St. Louis: Mosby; 2004;1043-74.
25. Reynolds MA, Chaves SS, Harpaz R, et al. The Impact of the Varicella Vaccination Program on Herpes Zoster Epidemiology in the United States: A review. The Journal of Infectious Disease. 2008;197:S224-7.
26. Brisson M, Edmunds WJ, Gay NJ, et al. Modeling the impact of immunization on the epidemiology of varicella zoster virus. Epidemiology and Infection. 2000;124:651-9.


