No matter the lens modality, discomfort is the leading cause of contact lens discontinuation, which only makes sense if you take a step back and think about it.1 However, what about when a patient needs or can benefit from the optics from a gas permeable (GP) lens? The comfort of a corneal GP or scleral lens for a patient is multifactorial. It isn’t always fit related, but one thing that is certain—if the edge configuration is not optimal, this will impact comfort and create lens awareness.
 |
|
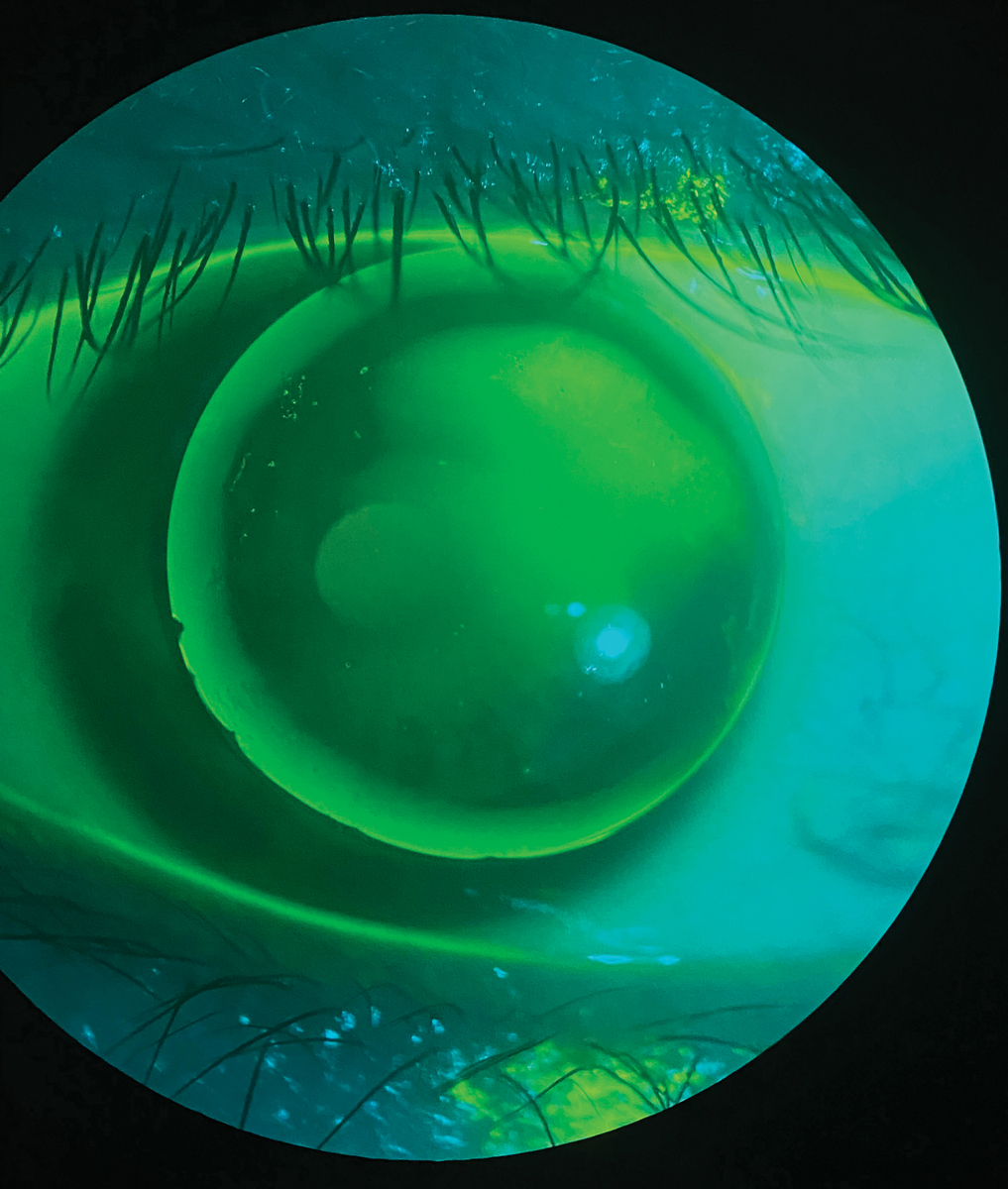
Fig. 1. A patient wearing a GP lens presenting with issues of severe discomfort and peripheral corneal staining from a chipped edge. Click image to enlarge. |
GP Lenses
Research shows that GP lenses can provide superior vision quality compared with soft lenses.2 Their smooth refracting surface with post-lens tear layer delivers crisp, stable optics, especially for patients with corneal astigmatism. Despite this advantage, practitioners still hesitate to fit these lenses, and patients who have had a negative experience are resistant to trying them again. When considering initial patient comfort, it’s no surprise why. The smaller diameter of GPs allow for greater movement on the eye, and the eyelid interaction with the edge of the lens causes lens awareness. Even though the majority of lens wearers prefer the initial comfort of a soft lens, it doesn’t mean they can’t be successful with GPs.3
With corneal GPs, the discomfort arises from the interaction between the rigid edge of the lens and the eyelids, particularly the upper lid margin. Edge shape and design are the most important parameters in initial lens comfort.4 The edge should be smooth and well rounded—in contrast, if a GP has a defective edge (chipped, abraded, blunt or sharp) the patient will be very aware of the lens with each and every blink, and it will make for quite the negative experience (Figure 1). A well-blended peripheral curve system is preferred to a poorly blended or unblended peripheral curve system, which can produce dryness, itchiness, and scratchiness.5 Fortunately, advances in computer-controlled designs and lathes have allowed modern GP lenses to be manufactured thinner with improved back surface geometry and more consistent edges.6
 |
|
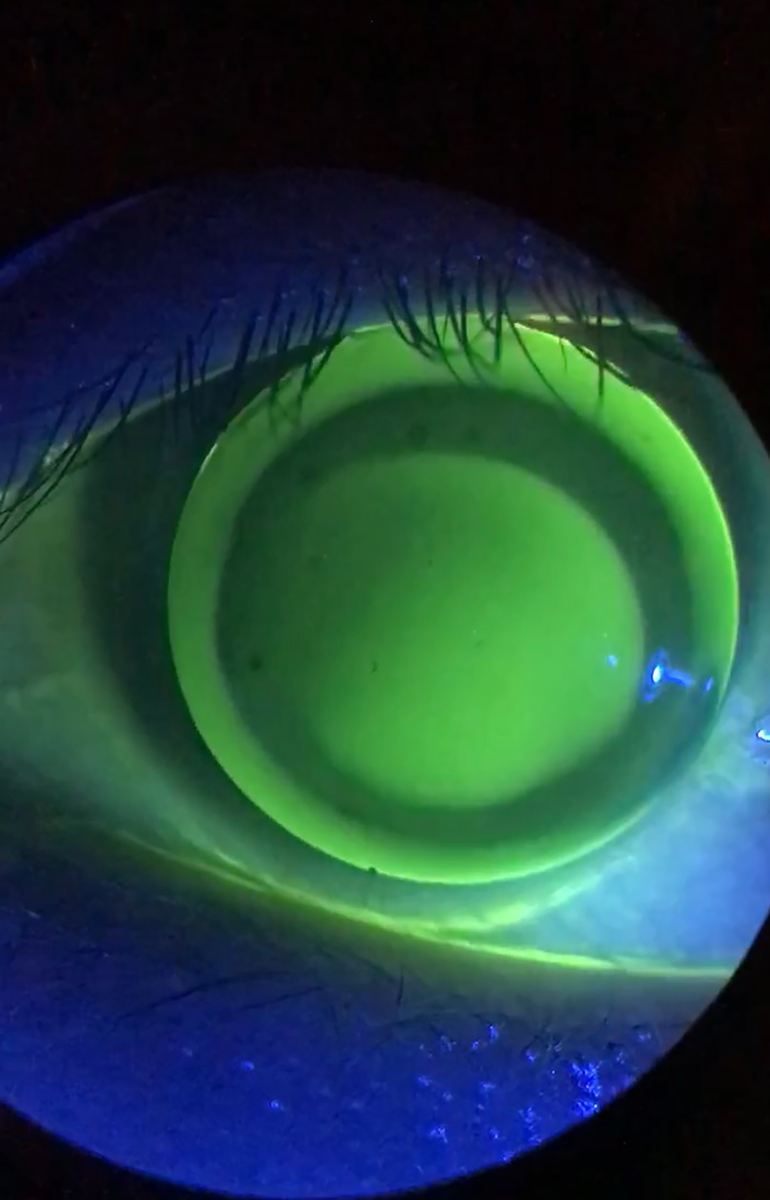
Fig. 2. A GP lens with excessive edge clearance, if not fixed will lead to three o’clock and nine o’clock staining and worse comfort for the patient. Click image to enlarge. |
GP Edge Factors
Three factors relating to the edge of the GP govern comfort: thickness and shape of the edge and the amount of edge clearance.7 A thin, tapered, rolled-edge design is desirable. It has been found that lenses with a well-rounded anterior edge profile versus a square anterior edge, are significantly more comfortable.8 One would think that a GP design with a thinner central thickness (CT), which results in a thinner edge thickness, would result in better comfort. However, believe it or not, one study actually found the opposite: thinner CT lenses were actually less comfortable than their stiffer counterparts, which was attributed to on-eye flexure or deformation of the lens during the blink cycle.9
One thing that always remains true, though, is that GP lens CT and edge thickness vary with changes in lens overall diameter (OAD) and with different lens powers.6 Edge thickness is greater in medium-to-high minus powers and thinner in low minus and all plus power lenses. As a result of the variation in edge thickness associated with lens powers, a lenticular design can be added to improve comfort: high minus lenses can benefit from plus lenticular design or an anterior center-near bevel, which will reduce the edge thickness and lessen lens awareness, inferior lens positioning and corneal desiccation from compromised blinking that usually results from a thick edge.
Typically, minus lens powers ≥-5.00D are lenticulated. Conversely, a minus lenticular design is used to increase the edge thickness to enhance lid interaction with the edge and minimize inferior decentration with minus powers ≤-1.50D and all plus power lenses. Avoiding an excessively thick edge can provide closer lid-to-cornea alignment and enhance patient comfort.10
Lastly, excessive corneal GP edge clearance can also be problematic for patient comfort. The greater the edge clearance, the greater the interaction with the eyelids and, in turn, the poorer the comfort.7 In addition, thick edge clearance can cause persistent discomfort because it funnels in surrounding tears, resulting in desiccation and corneal staining at three o’clock and nine o’clock on the peripheral cornea. This also affects the blink quality by an increased interaction between the lens edge and the superior lid, especially when the lens decenters (Figure 2).11 The best way to fix this is to either steepen the peripheral curve radius or decrease the peripheral curve width.
Conversely, if the peripheral curve is too steep to begin with—causing peripheral seal off—then corneal desiccation can occur, which can lead to vascularized limbal keratitis (VLK). This presents as a peripheral corneal lesion with superficial staining. More advanced cases can even lead to neovascularization and an elevated opacified region near the limbus where the edge of the lens was impinging on the peripheral cornea.12 A GP patient who has VLK will present with significant decrease in CL comfort and reduced wearing time, as well as sectoral limbal injection. This can be ameliorated by flattening the peripheral curve or the base curve if too steep or, in some cases, making the OAD to eliminate a steep fitting lens configuration.12
Although the initial comfort of corneal GPs may be a factor, if the fit and edge design are optimized and the patient is motivated, these lenses can be quite successful. However, if a patient isn’t up for the challenge, especially a contact lens neophyte, scleral lenses may be a better option.
 |
|
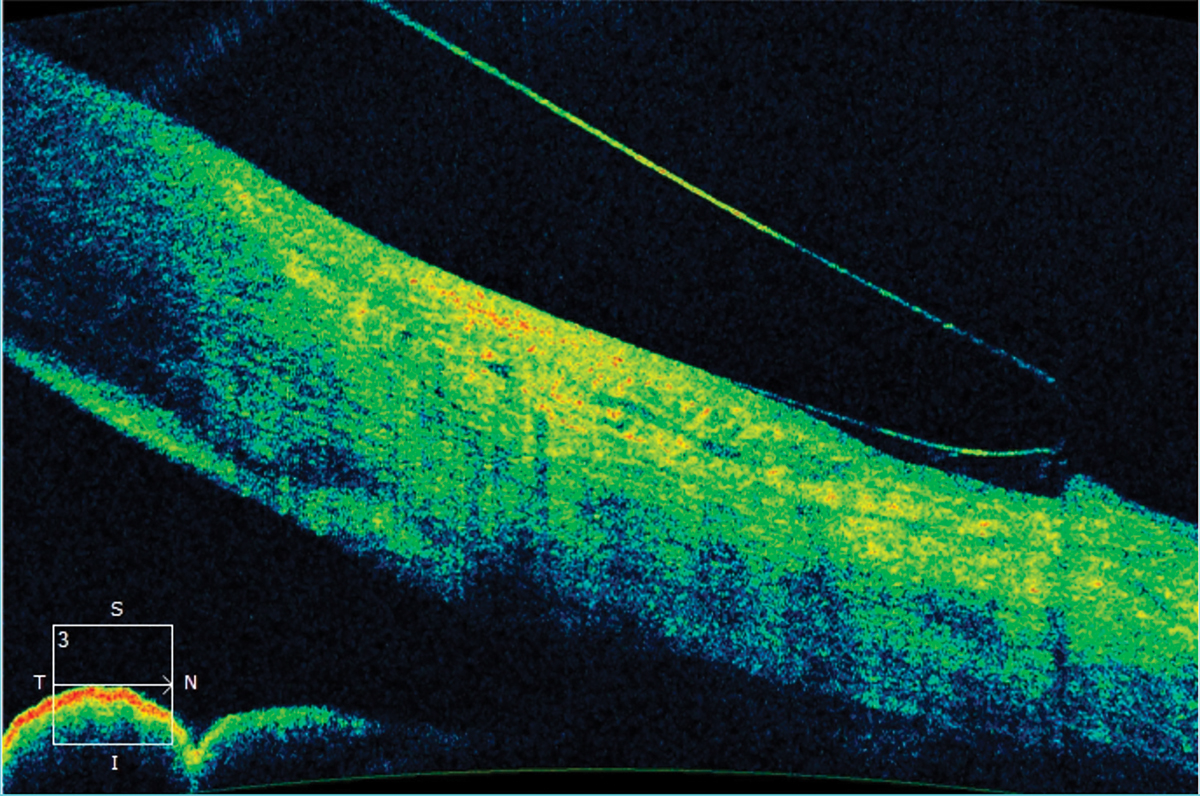
Fig. 3. Anterior segment OCT image showing edge lift of a scleral lens. This indicates a flat landing zone. A steeper landing zone was ordered to improve the haptic alignment. Click image to enlarge. |
Scleral Lenses
With a resurgence of sclerals in the last few decades, more patients than ever are wearing them. Indications for these lenses include corneal irregularity, ocular surface disease (i.e., keratoconjunctivitis sicca, Stevens-Johnson syndrome, graft vs. host disease) and refractive error. However, corneal irregularity is the most common.13 A recent study looked at scleral lens wear success over a 12-month period. During that time, 27% of patients discontinued lens wear. The two main reasons for discontinuation were handling issues (35%) and discomfort (19%).14
Discomfort can be multifactorial. One potential reason in scleral lens wearers is poor haptic or landing zone alignment to the conjunctiva and sclera. A steep landing zone will cause blanching of the conjunctival blood vessels at the edge of the lens and would require the optometrist to flatten the landing zone to better align to the conjunctiva. A flat landing zone will cause edge lift and possibly mid-haptic compression of the conjunctival blood vessels requiring a steeper landing zone (Figure 3).
These changes can be made to the whole lens if seen circumferentially. However, if seen in certain quadrants of the lens, a toric haptic design or quadrant specific design would lead to better alignment. Using an anterior segment OCT to evaluate the landing zone can be beneficial. The optimal lens edge to bulbar conjunctiva relationship demonstrates that 50% of the lens edge apex sinks softly into the conjunctiva and 50% of the edge apex is above the ocular surface.15
The Pacific University Scleral Shape Study showed that the sclera was nearly symmetrical at a chord length of 10.0mm to 15.0mm. However, the 15.0mm to 20.0mm chord was more asymmetrical. This data suggested that if the scleral lens diameter was 15mm or less, a spherical landing zone would likely be successful, whereas a scleral diameter larger than 15mm would benefit from a non-symmetric (toric or quadrant specific) scleral landing zone design.16 The Scleral Shape Study Group looked at the pattern of scleral shapes and found that a minority of patients (roughly one-third) had rotationally symmetric scleral shape patterns and the majority of patients (roughly two-thirds) had irregular patterns with either asymmetric depressions or elevations.17
So what can we take away from these studies? That when fitting scleral lenses, we should consider using toric or quadrant-specific haptics for larger diameter lenses. Using toric scleral landing zones in scleral lenses has been reported to improve comfort compared to spherical scleral landing zones.18 Interestingly, since these studies have been published, there has not been a shift toward fitting scleral lenses with toric or quadrant-specific landing zones. The majority of lenses being fit still have spherical landing zones.19
 |
|
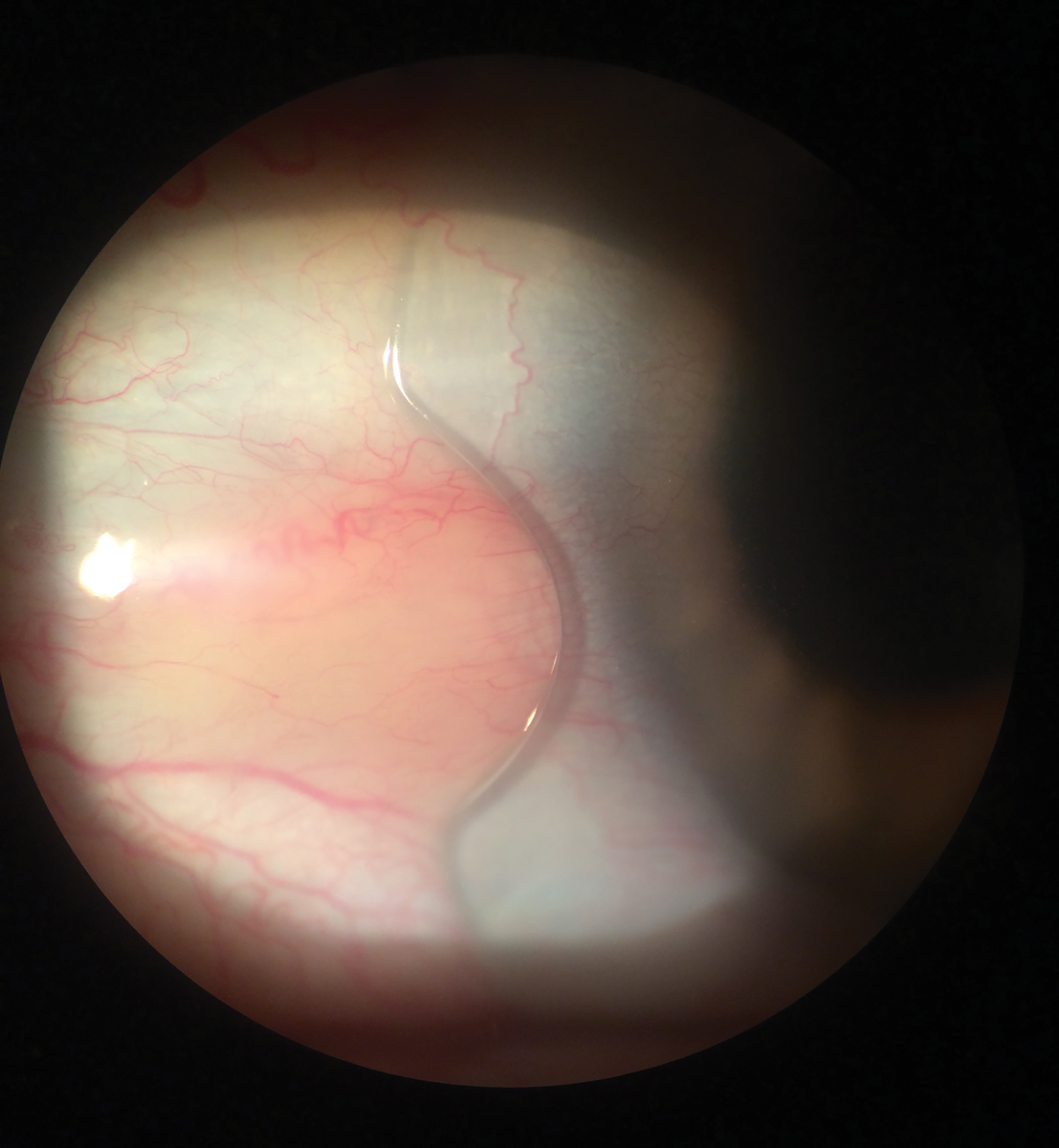
Fig. 4. Scleral lens with a notch to avoid interacting with a pinguecula. Click image to enlarge. |
Troubleshooting Sclerals
Discomfort during scleral lens wear can be attributed to multiple fitting issues, one of those being poor landing zone alignment to the conjunctiva. The following describes a few common problems encountered during the fitting process and the remedies that can lead to improved patient comfort and satisfaction.
Conjunctival elevations or obstacles should be taken into consideration when fitting a scleral lens. If the lens is interacting with a conjunctival obstacle, it can cause significant redness, discomfort and decreased wear time. Examples of these obstacles include pingueculae, pterygia, inclusion cysts and filtration blebs. Options to limit or avoid interaction with conjunctival elevations/obstacles include adjusting the diameter of the scleral lens, adding a notch to the edge of the lens, adding a focal area of increased elevation to the edge of the lens, using corneoscleral profilometry to design a freeform lens or impression-based fitting.
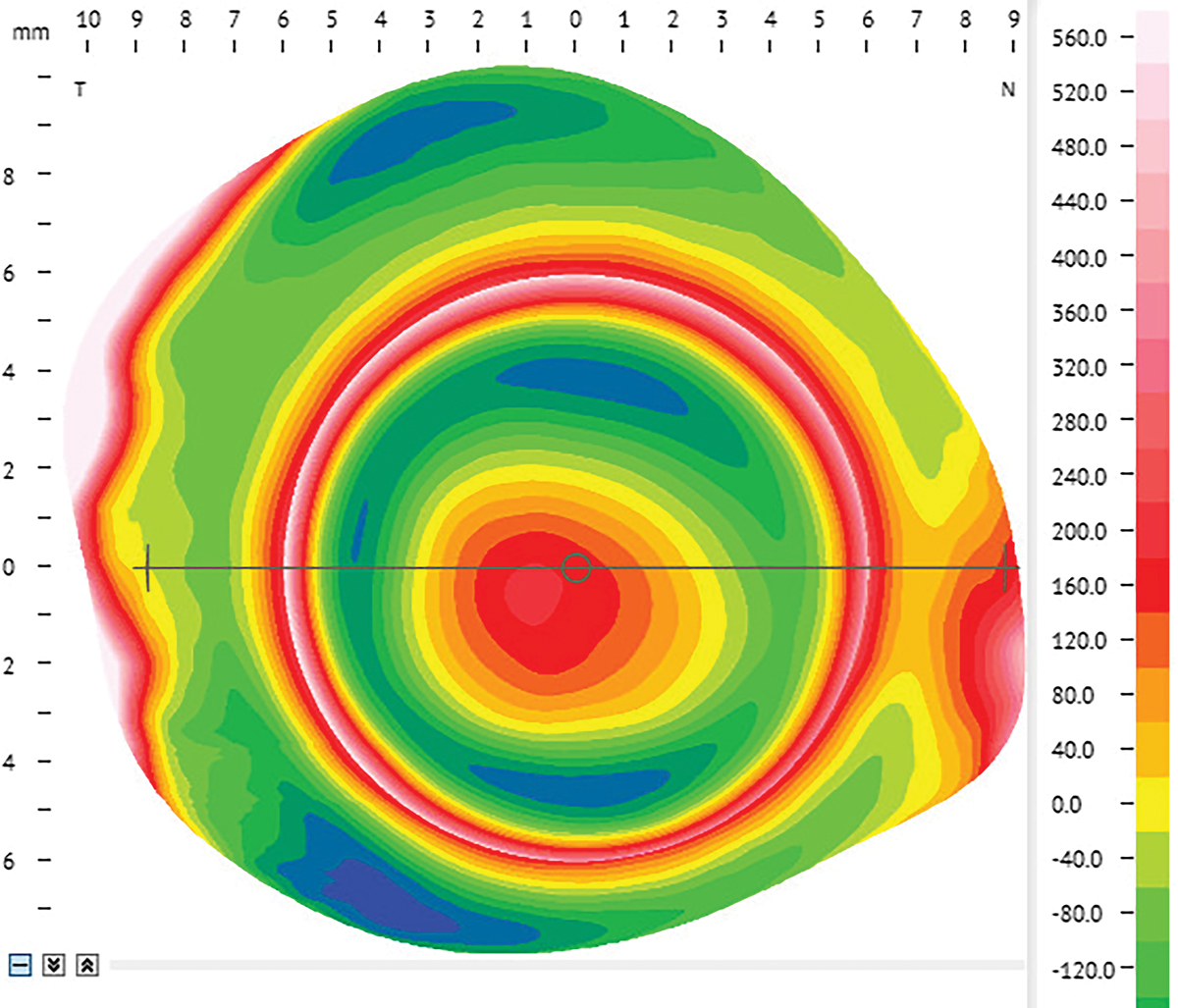 |
|
Fig. 5. Corneoscleral profilometry image OD of a patient with keratoconus and a nasal pinguecula. Click image to enlarge. |
If a conjunctival elevation is present and is located far enough away from the limbus, decreasing the diameter of the scleral lens would allow the haptic to land before the elevation. If the elevation was closer to the limbus, you could increase the diameter of the lens to land past the area of concern. When increasing the diameter of the lens, there will still be some interaction between the landing zone and the obstacle. This compression can lead to redness and lens awareness. If the elevation is not shallow enough, it could also cause edge lift, leading to bubble formation at the edge.
Adding a notch to the edge of the lens allows the lens edge to avoid the obstacle and fit around it (Figure 4). The information needed to design a notch is the location and size of the obstacle. The size of the obstacle will determine the width and depth of the notch. Before adding one, have a centered and stable lens, ideally with toric or quadrant specific haptics. It is recommended to order your first lens without a notch. This will allow you to determine the rotation and stability of the landing zones. If you have stable landing zones at the first follow-up visit, you can design the notch. One way to design the notch is by taking measurements with your slit lamp and taking pictures to send to the lab. Another way is by using a permanent marker to note on the lens the size and depth you want the notch to be. The final option is to create the notch yourself in the office if you have the appropriate tools.
Another option is adding a focal area of increased elevation at the lens edge. This will allow the lens to vault over the area of elevation and minimize the amount of interaction. The fitting process is similar to doing a notch. A stable lens fit with either toric or quadrant specific haptics is needed, along with the location and size of the obstacle. The only new piece of information needed is the height of the obstacle. You can obtain the height of the obstacle by either using corneoscleral profilometry, anterior segment OCT or estimating with the help of the lab consultants.
Corneoscleral profilometry provides detailed measurements of the cornea and sclera (Figure 5). This will allow you to have a better understanding of the scleral toricity prior to applying a diagnostic lens to the eye and help determine if spherical, toric or quadrant-specific landing zones are required. It will also show the location and measure conjunctival elevations. Freeform lenses designed from these machines can take into account conjunctival obstacles and include either a notch or focal area of elevation at the edge of the lens on the first lens order.
A final option to consider is using an impression-based method to design a freeform scleral lens matching the exact contour of the globe. Using an impression technique will expedite the fitting process for eyes that have complex conjunctival obstacles or that have failed traditional scleral lens wear. Although this is a more advanced technique, it has been shown to help those who have complex cases and leads to improved patient satisfaction.20
Takeaways
Whether it be a smaller corneal GP lens or a larger scleral lens, one thing is key: proper edge design is critical for comfort. When all else fails, the next time you have a patient expressing their dissatisfaction with their corneal GP or scleral lens comfort and the lens is centered with a good central fit, take a closer look at the edges, as that very well may be the key to success.
Dr. Andrzejewski practices at Chicago Cornea Consultants and serves as an adjunct assistant professor of optometry at the Illinois College Optometry as well as the Chicago College of Optometry. Her clinical work is dedicated exclusively to specialty contact lenses and surgical comanagement. She is associated with Bausch + Lomb Specialty Vision Products, Blanchard, Eaglet, Essilor Contacts, Ocular Therapeutix and SynergEyes.
Dr. Skoog is a clinical instructor at the Illinois College of Optometry in the cornea and contact lens department, where he previously completed a residency. He lectures on anterior segment disease and contact lenses. He has no disclosures.
1. Richdale K, Sinnott LT, Skadahl E, Nichols JJ. Frequency of and factors associated with contact lens dissatisfaction and discontinuation. Cornea. 2007;26(2):168-74. 2. Michaud L, Barriault C, Dionne A, Karwatsky P. Empirical fitting of soft or rigid gas-permeable contact lenses for the correction of moderate to severe refractive astigmatism: a comparative study. Optometry. 2009;80(7):375-83. 3. Fonn D, Gauthier CA, Pritchard N. Patient preferences and comparative ocular responses to rigid and soft contact lenses. Optom Vis Sci. 1995;72(12):857-63. 4. Edwards K. Rigid gas-permeable contact lens problem solving. Optician. 2000;219(5740):18-24. 5. Picciano S, Andrasko GJ. Which factors influence RGP lens comfort? Optom Today. 1989;4(5):31-3. 6. Bennett ES, Sorbara L, Kojima R. Gas-permeable lens design, fitting and evaluation. In: Bennett ES, Henry VA, eds. Clinical Manual of Contact Lenses. 4th Ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2014:112-56. 7. Jones L, Brennan NA, González-Méijome J, et al.; members of the TFOS International Workshop on Contact Lens Discomfort. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS37-70. 8. La Hood D. Edge shape and comfort of rigid lenses. Am J Optom Physiol Opt. 1988; 65(8):613-18. 9. Cornish R, Sulaiman S. Do thinner rigid gas permeable contact lenses provide superior initial comfort? Optom Vis Sci. 1996;73(3):139-43. 10. Bennett ES, Egan DJ. Rigid gas-permeable lens problem solving. J Am Optom Assoc. 2000;57(7):504-11. 11. Schnider CM, Terry RL, Holden BA. Effect of patient and lens performance characteristics on peripheral corneal desiccation. J Am Optom Assoc. 1996;67(3):144-50. 12. Grohe RM LK. Vascularized limbal keratitis. Internat Cont Lens Clin. 1989;16(7-8):197-209.. 13. Nau C, Harthan J, Shorter E , et al. (2023). Trends in scleral lens fitting practices: 2020 scleral lenses in current ophthalmic practice evaluation survey. Eye Contact Lens. 2023;49(2):51-5. 14. Macedo-de-Araújo RJ, van der Worp E, González-Méijome JM. A one-year prospective study on scleral lens wear success. Cont Lens Anterior Eye. 2020;43(6):553-561. 15. Barnett M, Fadel D. Clinical Guide for Scleral Lens Success. www.scleralsuccess.com. Accessed February 1, 2023. 16. Kojima R, Caroline P, Graff T, et al. Eye shape and scleral lenses—understanding the shape of the anterior segment can help improve success with lens design and fitting. CL Spectrum. www.clspectrum.com/issues/2013/april-2013/eye-shape-and-scleral-lenses. April 1, 2013. Accessed February 1, 2023. 17. DeNaeyer G, Sanders D, van der Worp E, et al. Qualitative assessment of scleral shape patterns using a new wide field ocular surface elevation topographer: The SSSG Study. JCLRS. 2017;1(1):12-22. 18. Visser ES, Visser R, Van Lier HJ. Advantages of toric scleral lenses. Optom Vis Sci. 2006;83(4):233-6. 19.. Schornack MM, Fogt J, Nau A, et al. Scleral lens prescription and management practices: Emerging consensus. Cont Lens Anterior Eye. 2023;46(1):101501. 20. Nau A, Shorter ES, Harthan JS, et al. Multicenter review of impression-based scleral devices. Cont Lens Anterior Eye. 2021;44(5):101380. |


