 Topical antibiotics revolutionized the way eye doctors care for patients with at-risk corneas. The ability to target infectious organisms with a site-specific therapy limited to the anterior segment of the eye improved clinical outcomes by orders of magnitude.
Topical antibiotics revolutionized the way eye doctors care for patients with at-risk corneas. The ability to target infectious organisms with a site-specific therapy limited to the anterior segment of the eye improved clinical outcomes by orders of magnitude.
Systemic medications would be impractical and ineffective in most ocular infections. Without topical therapies, many patients would suffer long-term visual and ocular health consequences.
Fluoroquinolone eye drops are now routinely and confidently prescribed as first-line therapy for a number of acute and chronic ophthalmic conditions, such as bacterial conjunctivitis, blepharitis and corneal disorders (e.g., abrasions, infiltrates, ulcers). They are also used routinely for prophylaxis, both pre- and post-ocular surgery.
Lest we take these workhorse drugs for granted, it’s important to review the history of the fluoroquinolone class and how each ‘generation’ has surpassed the previous one, then look to what’s ahead.
It Began By Accident
Prior to the introduction of fluoroquinolones, we simply had quinolones. These were the first antimicrobials to be developed in a lab. The first quinolone, nalidixic acid, was accidentally discovered in 1962 during the manufacture of the antimalarial compound chloroquine.
It was used systemically for the treatment of urinary tract infections, but the agent had little effect on gram-negative organisms. Alterations to its structure led to the development of second-generation products, such as oxolinic acid and clinoxacin. The use of these agents was also limited, due to poor systemic bioavailability and renal toxicity.1,2
The addition of fluorination to quinolones gave rise to the then-new class known as fluoroquinolones. The improved gram-positive activity, combined with solubility in ophthalmic solutions, expanded the clinical use of these drugs to the ocular field.3
The first fluoroquinolone, norfloxacin, introduced in 1978, resulted from the addition of fluorine at the C-6 position. It was the first fluoroquinolone to be used in the treatment of an ocular condition—namely, bacterial conjunctivitis. While norfloxacin exhibited excellent activity against gram-negative organisms, it proved to be largely ineffective against gram-positive bacteria.
How They Work
So, what exactly is the mechanism of action of the fluoroquinolones? Put simply, it is a bactericidal effect. Fluoroquinolones act in a unique manner: directly inhibiting DNA synthesis. This effect is mediated through the formation of a complex between the drug, the bacterial DNA and two essential bacterial enzymes (i.e., DNA gyrase and topoisomerase IV).4
DNA gyrase is an essential enzyme for DNA replication and transcription, while topoisomerase IV separates the interlocking of daughter DNA strands. If either of these processes is prevented, DNA and RNA synthesis and cell growth are blocked, which ultimately leads to cell death. Second- and third-generation fluoroquinolones preferentially inhibit one or the other, while the fourth-generation agents have the ability to exhibit balanced inhibition of both enzymes.5
In the early 1990s, the addition of a cylopropryl ring at the R1 position led to the development of the second-generation fluoroquinolone, ciprofloxacin, which had increased antibacterial activity against gram-positive and gram-negative pathogens.
Adding a pyridobenoxazine ring between the R1 and R8 positions led to the creation of another second-generation drug, ofloxacin.3 While many consider levofloxacin a third-generation fluoroquinolone, it’s really just a levo-isomer of ofloxacin and not a truly new molecular structure. In reality, its designation as third-generation fluoroquinolone is mostly due to timing: it was introduced nearly a decade after its most immediate predecessors.
A number of studies have demonstrated that, although these older-generation fluoroquinolones can provide coverage against the most frequently encountered gram-positive and gram-negative pathogens, there is increasing resistance to these agents—especially in gram-positive organisms, such as Staph. aureaus.6-8
Today’s Practice
The fourth-generation drugs, primarily used in modern clinical practice, are gatifloxacin and moxifloxacin. These entries are known for their enhanced activity against gram-positive organisms and atypical mycobacteria, improved drug delivery in the anterior segment and lower predisposition for selecting bacterial strains.9
The most recent entry to the fourth-generation class, besifloxacin, was introduced in 2009. This is the first product developed specifically for ocular use, with no systemic use to speak of; this is often cited as a beneficial hedge against the risk of antibiotic resistance. It is an entirely new chemical entity known as a chloro-fluoroquinolone, and appears to offer excellent potency against gram-positive and resistant strains.
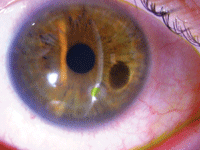 | |
|
A small, peripheral corneal ulceration. Fluoroquinolones are frequently prescribed to treat conditions such as corneal ulcers.
Photo: William Townsend, OD
|
Unlike other fluoroquinolones, which are manufactured in aqueous vehicles that are rapidly eliminated from the tear film, besifloxacin is a 0.6% suspension, formulated in a base designed to improve drug delivery in the tear film and prolong retention time on the ocular surface.10
The structural changes in the fourth-generation drugs confer less resistance potential than the earlier compounds.5,9 Additionally, these changes increase ocular tissue concentrations relative to organism minimum inhibitory concentration (MIC) to inhibit bacterial growth.5,9
Fourth-generation fluoroquinolones have been shown to be less prone to resistance to single-step mutations (which occur within genes encoding for one of the two principal target enzymes) that produce low-level resistance.11 This is accomplished by maintaining high mutant prevention concentration that is generally several fold higher than the MIC.11 Because these agents can inhibit both DNA gyrase and topoisomerase, they are unlikely to develop higher level, multi-step resistance.
Tomorrow’s Potential
Fourth-generation fluoroquinolones are safe, effective antibiotics that have greatly improved upon the capabilities of their predecessors. But further advances can still be made to future generations of these popular agents. A typical ‘wish list’ might include the following:
• improved bioavailability
• reduced dosage frequency to improve patient compliance
• reduced costs to insurers and patients
• the ability to eradicate infection faster than previous generations
• a lower potential risk of developing resistance
Given the improvements from the third to the fourth generation, it’s exciting to consider what the fifth generation may have in store!
1. Andriole V. The quinolones: past, present and future. Clin Infect Dis. 2005 Jul 15.41 Suppl2:S113-9.
2. Applebaum PC, Huber PA, The fluoroquinolone antibacterialss. Int J Antimicrob Agents 16:5-15, 2000.
3. Ball P, et al. Therapeutic advances of new fluoroquinolones. Exp Opin On Investig Drugs 1998 May;7(5):761-83.
4. Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg Inf Dis 2001;7:337-341.
5. Blondeau JM. Fluoroquinolones: mechanism of action. Surv Ophthalmol 2004;49(suppl 2):S73-S78.
6. Chaudhry NA, et al. Emerging ciprofloxacin resistant pseudomonas aeruginosa. AmJ Ophthalmol 1999:128:509-510.
7. Goldstein MH, et al. Fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalm 1999: 106:1313-8.
8. Asbell PA, et al. Ocular TRUST: Nationwide antimicrobial susceptibility patterns. Am J Ophthalmol 2008;145:951–958.
9. Hwang DG. Fluoroquinolone resistance in ophthalmology. Surv Ophthalmol 2004;49(suppl2):S79-S83.
10. Gardner S. Fluoroquinolone is developed expressly for ophthalmic use. Optometry Times, July 2009.
11. Hesje Ck, et al. MICs, MPCs and PK/PDs. Expert Rev Resp Med 2007;1:7-16.


