The cornea’s structure is critical to its abilities to refract light and prevent infection. However, it is often susceptible to many menacing threats. The stroma is the thickest layer of the cornea and plays a pivotal role in its stability. This article will present a review of the physiologic and biochemical bases for conditions affecting the corneal stroma that we often see in practice.
An Important Layer
The cornea is a clear, avascular tissue that covers about one-sixth of the circumference of the globe. It is responsible for two-thirds of the refraction of the eye. Its central thickness is, on average, 535μm, with the periphery being about 100µm thicker.1,2,3
The layers of the cornea are, from outermost to innermost: epithelium; anterior limiting lamina (Bowman’s membrane); stroma; posterior limiting lamina (Descemet’s membrane); and endothelium. The corneal stroma is a mesenchymal tissue, derived from mesoderm. At just over 500µm, it is the thickest layer of the cornea. In its periphery, it is continuous with the sclera.
It is a dense, very regular, connective tissue consisting of flattened, primarily type I collagen fibrils bundled together as lamellae 1µm to 2μm thick that are parallel to the corneal surface, creating the tissue’s transparency.4 There are approximately 242 lamellae in the human cornea.5 They are thinner and more interwoven in the anterior stroma and, as such, there are more of them there than in the posterior stroma. As a result, the anterior stroma is more rigid than the posterior. This plays an important role in corneal curvature and stability.6 It may also describe why one of the first signs of stromal edema—vertical striae—is always seen in the posterior stroma.1
One study demonstrated in vitro that the anterior stroma, 100µm to 120μm below Bowman’s layer, does not swell perceptibly when the cornea is immersed in water or saline for prolonged periods of time; this swelling takes place only in the posterior stroma, despite the fact that there exist negatively charged proteoglycans in both depths of the stroma.6
Keratocytes (fibroblasts) are the primary cells of the corneal stroma.7 In addition, neutrophils, lymphocytes, plasma cells, and/or histocytes may also be present in very small amounts.8 They are flat with various long processes extenuating from a central cell body in all directions.9 Located between the lamellae, they secrete an extracellular matrix (including collagen and proteoglycans) and produce crystalline proteins to help maintain the corneal transparency. The former can also assist in wound healing. Their other functions include assisting in stromal matrix turnover; interlamellar tethering (also helping to maintain transparency); intracorneal communication; and as a reservoir for glycogen (an energy source).1 There is also a ground substance composed of glycosaminoglycans (GAGs) and glycoproteins. The GAGs are hydrophilic and absorb fluid like a gel.10,11
 |
|
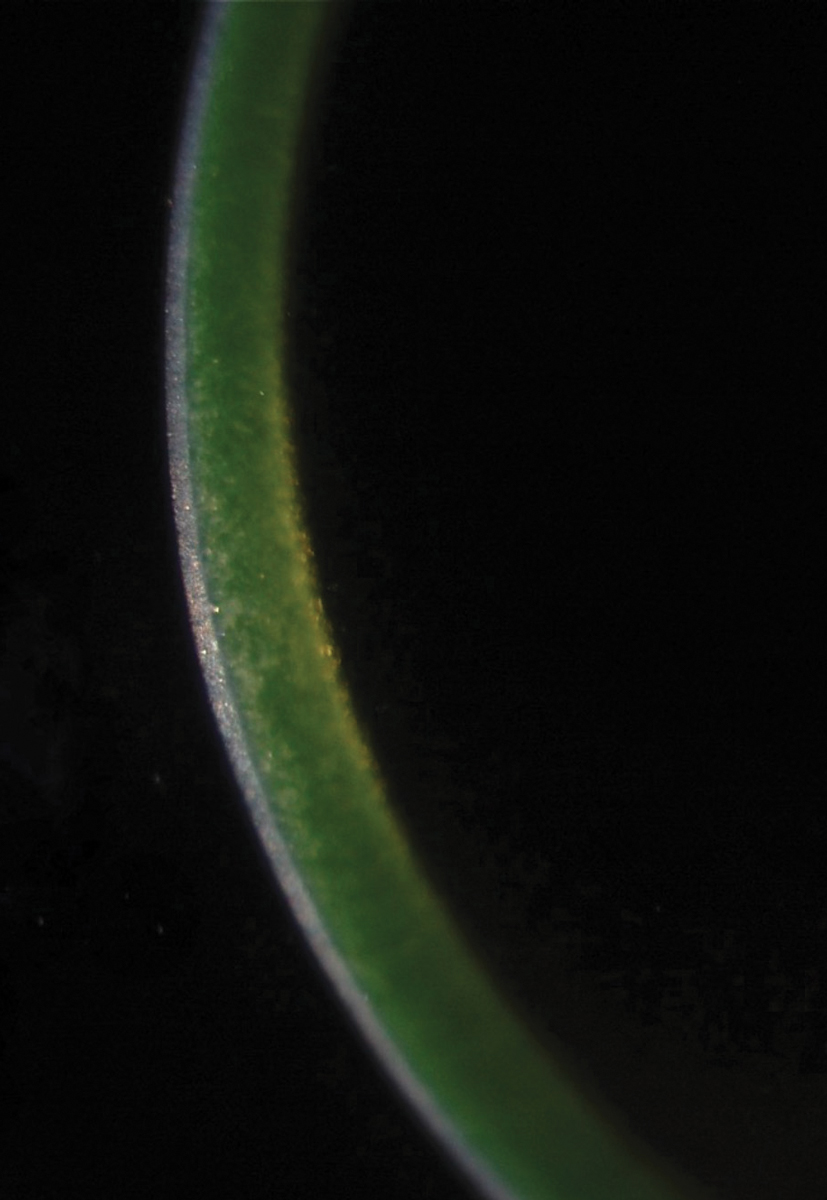
Fig. 1. The green glow of riboflavin indicates adequate penetration into the corneal stroma prior to CXL. Photo: Roy Rubinfield, MD. Click image to enlarge. |
Keratoconus
This noninflammatory corneal thinning disorder is characterized by a conical protrusion of the central or paracentral cornea. It is usually bilateral, and the thinning is most marked at the apex of the cone.3,12,13 While there is genetic predisposition, the exact etiology of keratoconus is an enigma, and it is difficult to diagnose in its earliest stages.13-15 It can be one of the most challenging conditions for eyecare providers to treat, as glasses and traditional soft contact lenses do not provide much benefit. Gas permeable (GP) contact lenses are the mainstay of treatment, and 10% to 25% of these patients will eventually require corneal transplantation, although this number is decreasing with the advent of scleral contact lenses and corneal collagen crosslinking (CXL).13,16-19
One author examined the keratoconic cornea histologically and wrote, “Corneal ectasia, a forward protrusion of the tissue, cannot be explained by simple loss of thickness […] other factors such as lamellar integrity are involved. In keratoconus, ectasia may occur in corneas which are within the normal thickness range […] thinning appears not to be the only contributing factor for this process […] (it) also involves biomechanical factors.”1
A sign of keratoconus—and a primary reason for vision loss and corneal transplantation—is stromal scarring. Another study found stromal scars in 24% of keratoconus eyes. They reported that the scars were always associated with compaction of stromal fibers and suggested that “stromal scarring may be the end-stage of the disarrangement of the stromal structure.”20
Corneal CXL and Haze
This procedure was FDA-approved in the United States for keratoconus in 2016 (iLink, Glaukos). It stiffens the cornea, minimizing the ectasia that can lead to vision loss. It uses oxygen, a photoenhancer (riboflavin) and ultraviolet light to induce collagen crosslinks (Figure 1). It locks the collagen fibrils together, increasing the thickness of the lamellae. Over 20 years of research confirms the benefits of this procedure.21,22
However, in the CXL method where the epithelium is removed prior to the procedure, 90% of patients had haze formation in the anterior to mid-stroma.23 This can resolve quickly, or may remain permanently, which may in rare cases affect visual acuity. This corneal haze appears as a dust-like change in the stroma or as a mid-stromal demarcation line. Its appearance after crosslinking has a different clinical appearance than haze after other procedures. Corneal haze after CXL was greatest at one month, plateaued at three months, and typically resolved by 12 months. The authors found that corneal haze is not correlated with loss of visual acuity.23
Corneal Transplantation
Over 10% of keratoconus patients will require corneal transplantation to remove the ectatic and scarred stroma.16,17 Historically, a full-thickness penetrating keratoplasty was performed. However, it was determined that by leaving the endothelium and Descemet’s membrane intact, the risk of allograft rejection diminished greatly. Years ago, performing a deep anterior lamellar keratoplasty produced inferior visual results; it also had more intra- and postoperative complications. However, with increased experience over the past decade, this has become the procedure of choice for many surgeons.24
Interestingly, the parallel alignment of the posterior lamellae of the stroma facilitates dissection in lamellar corneal grafting. This procedure is, however, not resistance-free, suggesting that there are elements which bind the collagen lamellae together. This is likely due to attachments between the collagen fibrils or the presence of other matrix proteins such as the proteoglycans or keratoepithelin.25
Patients who have undergone corneal transplantation (including those who have undergone endothelial transplantation) need to be monitored for graft rejection. Signs typically include stromal edema, new keratic precipitates on the endothelium, anterior corneal infiltrates and anterior chamber inflammation. Subepithelial infiltrates, epithelial line, conjunctival hyperemia or neovascularization may also be present. Treatment includes 1% prednisolone acetate q1h, with follow-up every three days. If the edema clears within one week, then diagnose rejection and keep the patient on a steroid dose greater than they were on prior to the episode. If the edema does not resolve, then diagnose corneal failure and refer for a regraft.26,27
 |
|
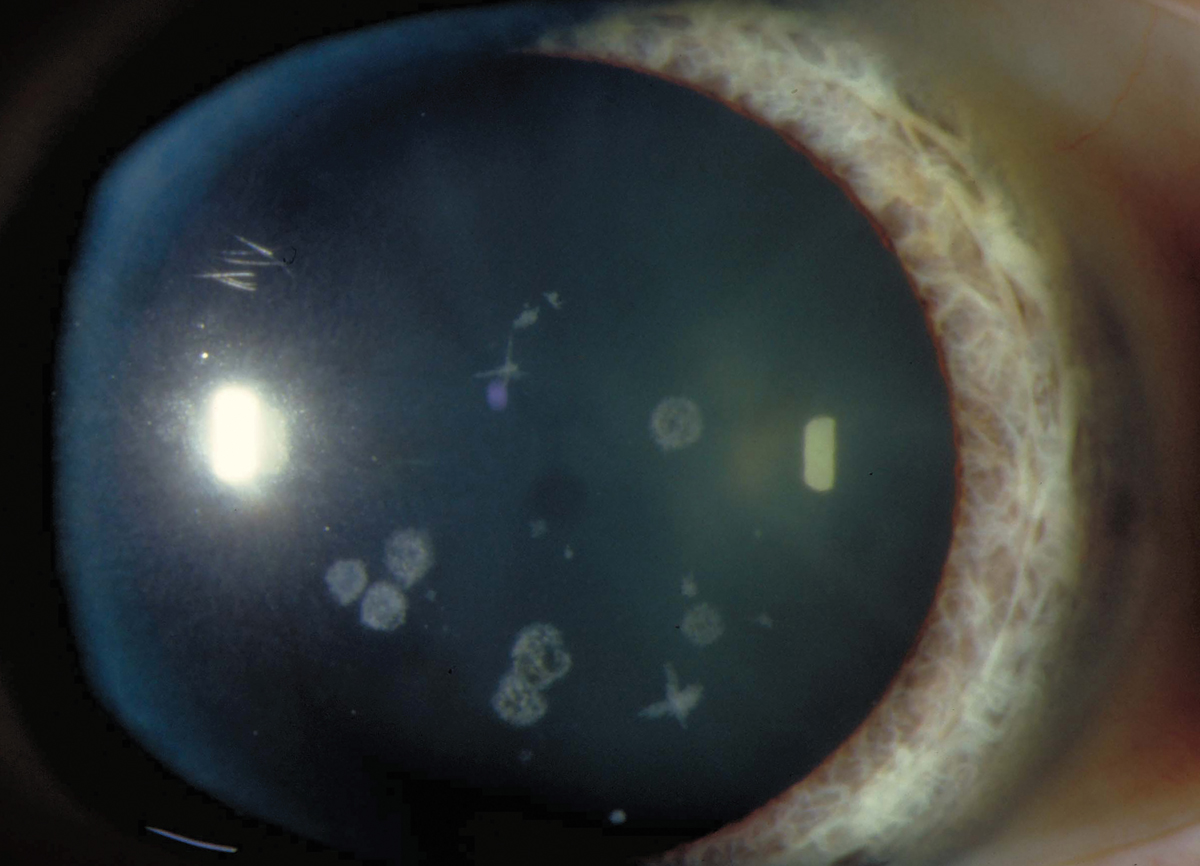
Fig. 2. Granular dystrophy in a 39-year-old woman. Click image to enlarge. |
Stromal Corneal Dystrophies
A corneal dystrophy is bilateral, symmetric, centrally located, avascular and hereditary (typically autosomal dominant and usually unrelated to systemic disease). The newest classification of the corneal dystrophies comes from the International Committee for Classification of Corneal Dystrophies, as described in Cornea in 2015.28 They are divided into categories based on the layer(s) of the cornea that are involved: (1) epithelial and subepithelial, (2) epithelial-stromal transforming growth factor beta-induced, (3) stromal and (4) endothelial
Some of the more common corneal dystrophies involve the stroma:
Granular dystrophy, type 1 (Figure 2):
- White opacities in superficial stroma of central cornea
- 7th-8th decade: opacities enlarge, coalesce and deepen
- Hyaline
- Good visual since the stroma between the lesions remains clear
Lattice dystrophy:
- Refractile lines, anterior-to-mid-stromal dots and faint central haze, subepithelial round opacities
- Stroma between the lesions remains clear until later in life
- Amyloid
Granular dystrophy, type 2 (formerly Avellino dystrophy):
- Granular (early onset) and lattice (later) changes in same eye
- Hyaline and amyloid
Macular dystrophy:
- Diffuse, grayish-white spots in central portion of anterior stroma 3rd decade: extends to endothelium and limbus Descemet membrane opacified and endothelial guttata
- Mucopolysaccaride
Schnyder (crystalline) dystrophy:
- Round, oval, discoid or annular central opacity composed of needle-shaped crystals, sometimes with arcus and limbal girdle
- Cholesterol
Fleck dystrophy:
- Small, discrete, dandruff-like specs in the stroma that extend to the periphery
- Visual acuity good
The stromal lesions may induce irregular astigmatism. Initially, GP (corneal or scleral) lenses may correct the irregular astigmatism created by the lesions. If the dystrophy worsens—and it is anterior enough in the stroma—phototherapeutic keratectomy may be performed to ablate the deposits. Later, if the deposits create even more serious vision loss, a corneal transplant may be performed.29
 |
|
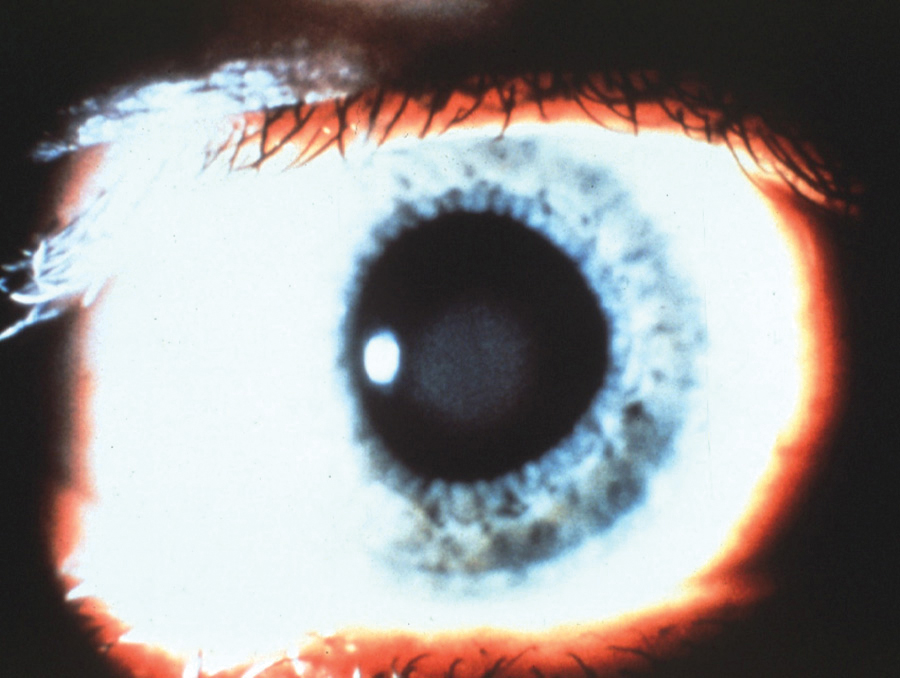
Fig. 3. Central corneal clouding caused by PMMA lens wear. Photo: Lisa Badowski, OD, MS. Click image to enlarge. |
Stromal Edema
If the epithelial or endothelial barrier is damaged, or the endothelial pump compromised, there will be an uptake of water into the stroma, resulting in swelling. The loss of the endothelial barrier results in a much greater corneal thickness increase than the loss of the epithelial barrier. The corneal stroma swells due to the hypertonic nature of the collagen, salts and proteoglycans. With corneal edema also comes a loss of proteoglycans.11
Histologically, in a swollen cornea the collagen fibrils are no longer of equal distance from each other. This causes a loss of transparency.11
Contact lens wear. The avascular cornea typically receives its oxygen from the atmosphere. However, the hypoxia created by lens wear can create stromal edema. Central corneal clouding could occur after just a few hours of wearing the lenses (Figure 3). Hallmark studies taught practitioners and material manufacturers alike which materials, lens thicknesses and wearing schedules were necessary to prevent corneal edema.
So, what is the mechanism whereby hypoxia causes stromal swelling? The lack of oxygen causes metabolic changes that can affect the epithelium, limbus, stroma and endothelium. Stromal swelling, in particular, results from increased lactate content following hypoxia.10
Patients often overwear their lenses and sleep in them, creating hypoxic conditions. Failing to clean them properly results in deposits on the lenses that can prevent the passage of oxygen to the cornea. Scleral GP contact lenses are three times the thickness of corneal GP lenses and cover the entire cornea. Corneal GPs allow oxygen to pass around the contact lens to the peripheral cornea. The oxygen needs to pass through the under-lens tear layer, and the thicker the layer the less readily oxygen will pass through. Much research has been conducted on this topic to ensure that these fantastic lenses for correcting corneal irregularity are not causing other problems for our patients. However, some of today’s lenses are still too thick, and the tear layers too deep, to prevent corneal edema during lens-wearing hours.30
Infectious keratitis (corneal ulcers). This is often categorized as Acanthamoeba/parasitic, bacterial, fungal or herpetic. Treatment needs to be quick and aggressive to avoid the corneal scarring that can lead to corneal transplantation.
Aphakic/pseudophakic bullous keratopathy. Corneal edema can occur in eyes after the intraocular lens has been removed. This may be caused by a preferential loss of anterior stromal keratocytes.25 Additional signs include corneal bullae, Descemet’s folds, corneal neovascularization, endothelial guttata, elevated eye pressure and/or cystoid macular edema (CME). Treatment includes topical sodium chloride 5% drops QID and ointment at night. If the bullae rupture, use a bandage contact lens to facilitate healing and use an antibiotic to prevent infection. The elevated eye pressure and/or CME will need to be treated as well.31
Other etiologies. Stromal edema can also be found in congenital glaucoma, angle-closure glaucoma, birth trauma (forceps injury), corneal abrasions and other corneal injuries.
Takeaways
The corneal stroma is a remarkable structure. It is imperative to maintaining corneal clarity and sturdiness. As practitioners, it is important for us to help our patients maintain this transparency so that they can maximize their vision at all times.
Dr. Gromacki is a fellow of the American Academy of Optometry and a diplomate in the Cornea, Contact Lens and Refractive Technologies section. She serves as the director of the contact lens service at a subspecialty group practice in Maryland. In the past year, she has received consulting fees from Alcon, Avellino, Bausch + Lomb SVP, Glaukos, Johnson & Johnson Vision Care, Lentechs, Pentavision, RVL Pharmaceuticals, Santen and Tarsus; and speaking fees from Alcon, Bausch + Lomb SVP, CooperVision SEC, GPLI, Glaukos, PSS Eyecare, Santen and Johnson & Johnson Vision Care.
1. Bergmanson JPG. Cornea. In: Bergmanson J, ed. Clinical Ocular Anatomy & Physiology. 29th Edition. Texas Eye Research and Technology Center; 2022. 2. Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44(5):367-408. 3. Gromacki SJ, Barr JT. Central and peripheral thickness in keratoconus and normal patient groups. Optom Vis Sci, 1994;71(7):437-41. 4. Garcia M, Horne J, Gondo M et al. Further assessments of the lamellae of the human corneal stroma by transmission of electron microscopy. Optom Vis Sci. 2003;80(12):S158. 5. Bergmanson JPG, Horne J, Doughty M et al. Assessment of the number of lamellae in the corneal region of the normal human corneal stroma at the resolution of the transmission electron microscope. Eye Contant Lens. 2005;31(6):281-7. 6. Müller LJ, Pels E, Vrensen GFJM. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85(4):437-43. 7. Clareus F. Thesis. Uppsala University Medical School, E. Westrell; Stockholm: 1857. Hornhinnans histologi. 8. Kuwabara T. Current concepts in anatomy and physiology of the cornea. Contact Intraocul Lens Med J. 1978;4:101-32. 9. Gipson IK. Anatomy of the conjunctiva, cornea, and limbus. In: The Cornea, Smolin G, Thoft RA, eds. 3rd Edition, Little, Brown and Company, New York, NY; 1994:10. 10. Bonnano JA. Corneal edema. In: Silver JA, ed. Anterior Segment Complications of Contact Lens Wear. Churchill Livingstone, New York, NY; 1994:16-26. 11. Edelhauser HF, Geroski DH, Ubels JL. In: Smolin G, Thoft RA, eds. The Cornea. 3rd Edition, Little, Brown and Company, New York, NY; 1994;25-9. 12. Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28(4):293-322. 13. Gromacki SJ. What every optometrist should know about keratoconus. Contemporary Optometry. 2003;1(6):1-8. 14. Szczotka LB, Barr JT, Zadnik K. A summary of the findings of the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: CLEK Study Group. Optometry. 2001;72(9):574-84. 15. Gromacki SJ, Seligson V. Diagnosing keratoconus without a topographer. Optom Man 2014;49(6):47-50. 16. Kennedy JH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267-73. 17. Lass JH, Lembach RG, Park SB et al. Clinical management of keratoconus: a multicenter analysis. Ophthalmology. 1990;97(4):433-45. 18. Ling JL, Mian SI, Stein JD et al. Impact of scleral contact lens use on the rate of corneal transplantation for keratoconus. Cornea. 2021;40(1):39-42. 19. Godefrooij DA, Gans R, Imhof SM, Wisse RP. Nationwide reduction (Netherlands) in the number of corneal transplantations for keratoconus following the implementation of crosslinking. Acta Ophthalmol. 2016;94(7):675-8. 20. Fernandes BF, Logan P, Zajdenweber ME, et al. Histopathological study of 49 cases of keratoconus. Pathology. 2008;40(6):623-6. 21. Hashemi H, Seyedian MA, Miraftab M, et al. Corneal collagen crosslinking with riboflavin and ultraviolet A irradiation for keratoconus: long-term results. Ophthalmology. 2013;120(8):1515-20. 22. Wittig-Silva C, Chan E, Islam FM, et al. A randomized, controlled trial of corneal collagen crosslinking in progressive keratoconus three year results. Ophthalmology. 2014;121(4):812-21. 23. Greenstein SA, Fry KL, Bhatt J, et al. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010;36(12):2105-14. 24. VanDijk K, Baydoon L, Konder R et al. Contact lenses after keratoplasty. CL Spectrum. 2014; 29(8):36-42. 25. Bron AJ. The architecture of the corneal stroma. Br J Ophthalmol. 2001;85(4):379-83. 26. Ehlers JP, Shah CP, eds. Corneal graft rejection. In: The Wills Eye Manual 5th Edition. Wolters Kluwer. 2008;97-8. 27. Bronner A. Was it a failure or rejection? RCCL. 156(1):36-7. 28. Weiss JS, Møller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies—edition 2. Cornea. 2015;34(2):117-59. 29. Catania L. Diagnoses of the Cornea. In: Primary Care of the Anterior Segment. Appleton & Lange, Norwalk, CT; 1988:90-1. 30. Michaud L, Van der Worp E, Brazeau D, et al. Predicting estimates of oxygen transmissibility for scleral lenses. Cont Lens Anterior Eye. 2012;35(6):266-71. 31. Ehlers JP, Shah CP, eds. Aphakic bullous keratopathy/pseudophakic bullous keratopathy. In: The Wills Eye Manual 5th Edition. Wolters Kluwer; 2008:95-6. |


