Some of these corneal alterations are transient, such as endothelial blebs, edema, limbal injection, myopic creep, epithelial microcysts and epithelial thinning.1-5
Although these conditions tend to be reversible, they each resolve at differing rates. Examples of non-reversible complications include corneal vascularization, polymegethism and pleomorphism.6
Silicone hydrogel (SH) lenses, introduced in the late 1990s, were designed to decrease the risks of hypoxia-related complications.
In 2001, Covey et al. determined that SH wear actually had the same physiological effects as not wearing a lens at all.7 A recent literature review suggests that SH materials have effectively eliminated hypoxia for most patients.8
Certainly, SH materials do eliminate hypoxia-induced complications, and while these materials make up the majority (64%) of the US contact lens market, there are still about 12 million people wearing either hydrogel lenses, GP lenses or hybrids.7,9
 | |
|
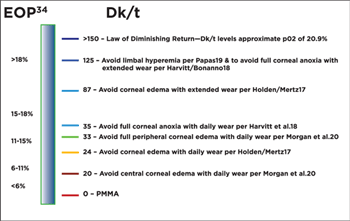
Figure 1. Dk/t levels necessary to mitigate hypoxic stress.
|
Advances in SH materials continue to occur, and these materials are available in sphere, toric and multifocal designs and in multiple replacement modalities.
Custom SH lenses and hybrid lenses equipped with SH soft skirts are also available.
Silicone hydrogels are also becoming more readily available for individuals with high ametropia.
For conditions such as high astigmatism and individuals who do not adapt well to SH materials, hydrogels remain the only readily available soft lens option.
Previous-generation hydrogel materials still have a role in the market; however, individuals wearing these materials may be at increased risk for hypoxia-related complications.
Additionally, a lens manufactured out of SH may give the practitioner a false sense of security about its ability to reduce or eliminate hypoxia.
Permeability vs. Transmissibility
The ability of oxygen to pass through a contact lens polymer has been evaluated with various techniques, each of which has limitations. These measures include equivalent oxygen percentage (EOP), oxygen flux, oxygen consumption and the measure most familiar to clinicians, oxygen permeability (Dk).
Briefly, EOP is a measure of oxygen uptake rate of the cornea immediately after a lens is removed. Oxygen flux is the amount of oxygen that reaches the eye, while oxygen consumption is the amount of oxygen the cornea consumes under a specific condition.
With Dk, the diffusion coefficient (D) and the oxygen solubility (k) form an inherent oxygen permeability material property. The methodology of determining the Dk differs; however, each lens material does have a published Dk value provided by manufacturers.10
A more critical value for the clinician is oxygen transmissibility (Dk/t). Transmissibility takes into account the thickness of the contact lens and is measured in units x 10-9 (cm/s)(mlO2/ml x mm Hg). Benjamin has referred to these units as Fatt Dk/t units (honoring the pioneering work of Irving Fatt in this field of study).11
Critical Oxygen Transmissibility Levels
The earth’s atmosphere is 20.9% oxygen, and because the cornea is avascular, it receives the majority of its oxygen from the atmosphere, with lesser amounts received from the aqueous humor and limbal vasculature.12 The corneal tissues use oxygen at differing rates, with the epithelium metabolizing oxygen 10 times faster than the stroma.13
If a contact lens is placed on the cornea that does not allow an EOP of 20.9%, the cornea theoretically suffers from hypoxia and will thus experience physiological alterations. Brennan has evaluated oxygen flux, which is the volume of oxygen reaching an area of the corneal surface over a period of time.14,15
|
|
|
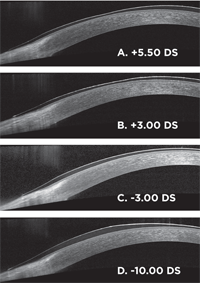
Figure 2. Thickness profile for soft contact lenses of various powers (A-D). Each image is half a spherical lens from the approximate optic center to the edge. Center thickness of the -3.00 DS lens is 70µm.
|
Numerous investigators have performed substantial research evaluating different theories on how oxygen traverses through a contact lens polymer. Others have evaluated EOP.16
However, much of the research has been dedicated to oxygen transmissibility and the Dk/t levels necessary to mitigate hypoxic stress. In 1984 Holden and Mertz found that a Dk/t of 24 was necessary to avoid corneal edema for daily wear, and a Dk/t of 87 was necessary for extended wear.17
Harvitt and Bonanno found Dk/t levels of 35 and 125 were necessary to avoid anoxia throughout the entire corneal thickness for open and closed eye conditions, respectively.18
Papas found that a Dk/t of 125 was necessary to avoid limbal injection.19
Morgan and Efron stated that Dk/t levels need to be approximately 20 and 33 for the central and peripheral portions of a soft lens, respectively, in order to avoid edema with daily wear.20
These Dk/t levels are summarized in figure 1.
Lens Transmissibility
Most manufacturers list Dk/t for specified lens powers. For instance, senofilcon A has a Dk of 103. At -3.00D, the lens has a published center thickness of 0.07mm, and the associated central Dk/t is 147. At +3.00D, the center thickness is 0.147mm, with a central Dk/t of 70.
The initial value of 147 exceeds the original Holden-Mertz criteria of 87 in order to limit overnight corneal edema to approximately 4%, while the +3.00D fails to reach 87. Towards the edge of the optic zone (approximately 4mm from the optic center) of a +3.00D lens, the Dk/t will exceed 87.
Contrary to a plus power lens, a high minus lens will have an increased thickness towards the optic zone edge, and therefore, depending on the power, may not be able to achieve the desired Dk/t of 87.
Using a Spectralis OCT (Heidelberg Engineering), figure 2 demonstrates the thickness profiles for various powers of balafilcon lenses. Since the lens thickness can dramatically change depending on power or design, such as the ballasting technique for toric lenses, central Dk/t is misleading.
Benjamin has proposed that mean harmonic thickness may be a much more valid value, as it averages the radial thickness of the center and periphery of a lens.21
Transmissibility with Specialty Lenses
The practitioner needs to pay much closer attention to the Dk/t for patients wearing latheable SH custom lenses, piggyback fits, scleral lenses and orthokeratology lenses.
Custom SH lenses are currently being manufactured with efrofilcon A (Definitive; Dk = 60, Contamac US). Although this material is silicone hydrogel, it is oftentimes used for high ametropia, resulting in rather thick portions of contact lenses.
An example is for the correction of keratoconus, which tends to require relatively thick soft lenses, typically ranging from 0.3mm to 0.6mm.22 Oxygen transmissibility with efrofilcon A will then range from 10 to 20 units.
A Dk/t of 10 should theoretically result in corneal edema with daily wear; however, reports state that hypoxia-related complications with these lenses are actually quite rare. This may be due to the amount of lens movement associated with the blink, which allows for increased tear exchange with oxygen-rich tears.23
Piggyback fitting is often reserved for patients with irregular corneas who suffer from GP lens awareness, or to assist in stabilizing the GP lens.
If the practitioner determines that a piggyback fit is the most appropriate option, extra attention must be paid to material selection. Ideally, the soft lens will be a low-plus SH that will provide acceptable transmissibility, while the GP lens should have a Dk of 100 or greater (Table 1).
|
Table 1. Currently Available GP Materials with Dk Value of 100 or Greater
| |||
|
Brand
|
Manufacturer
|
Dk
|
|
|
Boston XO
|
Bausch+Lomb
|
100
|
|
|
Boston XO2
|
Bausch+Lomb
|
141
|
|
|
Optimum Extra
|
Contamac U.S.
|
100
|
|
|
Optimum Extreme
|
Contamac U.S.
|
125
|
|
|
Menicon Z
|
Menicon America Inc.
|
163
|
30 day CW
|
|
Paragon HDS 100
|
Paragon Vision Sciences
|
101
|
6 night/7 day EW
|
Historically, the soft lens has been a low-powered plus lens, while a recent report suggests the use of minus-power lenses. 24,25 Either way, the soft lens and GP should be of high Dk. The Dk/t of the piggyback system can be derived using an equation, which can be found below.26
Recently, there has been an increase in use of scleral lenses for irregular corneal conditions or ocular surface disease. These lenses provide good comfort, vision and therapeutic benefit with many ocular surface conditions.
Unlike corneal GP lenses, scleral lenses rest on the conjunctiva and, if fit appropriately, will completely vault the corneal surface. The thickness of the corneal vault and the lack of tear exchange must be taken into consideration when assessing the risk of hypoxia with scleral lenses.
In 2012 Michaud calculated the theoretical oxygen transmissibility of scleral lenses in conjunction with the thickness of the tear reservoir.26 He proposed the following equation:
To perform this calculation, it is first necessary to know the Dk of the tears, which has been determined to be approximately 80.27 The center thickness of a scleral lens is related to its overall diameter (OAD).
For instance, an 18mm OAD lens may have a center thickness of approximately 300µm, while a 24mm OAD lens may have a center thickness of 500µm. If a patient is wearing a lens with a 300µm center thickness and a Dk of 100 fit with a 250µm vault, the calculated transmissibility of the system would be 16.
This Dk/t would not meet the Holden-Mertz or Morgan et al. criteria, and in theory, this would put the patient at increased risk of corneal hypoxia. Michaud explains, however, that hypoxia-related complications with scleral lenses are fortunately not common events.
Lenses for orthokeratology fits should be manufactured out of high Dk GP materials. Typically, the Dk of these lenses is over 100. Using a thickness gauge, a sample of orthokeratology lenses yielded center thicknesses of approximately 200 to 220 microns.
Therefore, the central Dk/t of a 100 Dk lens would be approximately 50. Using Menicon Z (Dk = 163) would yield a central Dk of approximately 80, which approaches the Holden-Mertz criteria. Fortunately, corneal edema is not commonly found in patients wearing ortho-K lenses.
Soft Lenses with High Transmissibility
Many SH lenses with high Dk values have received FDA approval for six-night/seven-day extended wear or 30-day continuous wear (Table 2). Though the Dk/t differs with lens design and power, the FDA approval covers the material across the entire power range.
|
Table 2. Currently Available Silicone Hydrogel Brands with Extended Wear or Continuous Wear FDA Approval
| ||||
|
Brand
|
Manufacturer
|
Dk
|
Replacement
|
Overnight Approval
|
|
Air Optix Night & Day
|
Alcon
|
140
|
Monthly
|
30 day CW
|
|
Purevision & Purevision2
|
Bausch+Lomb
|
91
|
Monthly
|
30 day CW
|
|
Biofinity
|
CooperVision
|
128
|
Monthly
|
6 night/7 day EW
|
|
Air Optix
|
Alcon
|
110
|
Monthly
|
6 night/7 day EW
|
|
Acuvue Oasys
|
Vistakon
|
103
|
2-Week
|
6 night/7 day EW
|
Soft lens manufacturers have also introduced SH materials in daily disposable designs. The first such lens option was the Acuvue Trueye (Vistakon). For a number of years, the Trueye was available in the US as narafilcon B with a Dk of 50, while the rest of the world had access to narafilcon A (Dk/t @ -3.00D of 118), which was only recently made available in the US market.
The year 2013 also saw the arrival of a new SH, the Dailies Total1 (Alcon). This novel material places SH (delefilcon A) in between high water content anterior and posterior lens surfaces, which creates a gradient water content lens. This lens has a Dk/t @ -3.00D of 156.
Although not a SH material, the Biotrue 1-Day, trademarked as a “Hypergel,” consists of 78% water--equal to the water content of the cornea. At -3.00D this lens has a Dk/t of 42.
All three of the previously discussed daily disposable lens materials offer Dk/t values that exceed the Holden-Mertz criteria for daily wear, and are indications that manufacturers are developing products that are intended to increase comfort and reduce hypoxia.
Silicone hydrogel and high Dk GP materials have been extremely important advances in contact lenses. Although these materials essentially eliminate adverse effects secondary to hypoxia, they have not been associated with a decrease in the incidence of microbial keratitis, and have actually been associated with an increased risk of infiltrative keratitis.28-33
Corneal hypoxic changes are becoming much less common; however, when fitting patients with high ametropia or a specialty fit, the clinician needs to be knowledgeable of the material Dk and the thickness of the lens or lens system.
Dr. Zimmerman is an associate professor of clinical optometry at The Ohio State University College of Optometry. He has articles published in a number of journals, including Archives of Ophthalmology, Optometry, Human Factors and Ophthalmic Epidemiology.
1. Holden BA, Williams L, Zantos SG. The etiology of transient endothelial changes in the human cornea. Investigative Ophthalmology & Visual Science 1985;26:1354-9.
2. Papas EB. The role of hypoxia in the limbal vascular response to soft contact lens wear. Eye & Contact Lens 2003;29:S72-4.
3. Dumbleton KA, et al. Changes in myopic refractive error with nine months’ extended wear of hydrogel lenses with high and low oxygen permeability. Optometry and Vision Science 1999;76:845-9.
4. Keay L, et al. Microcysts: clinical significance and differential diagnosis. Optometry 2001;72:452-60.
5. McNamara NA, et al. Soft lens extended wear affects epithelial barrier function. Ophthalmology 1998;105:2330-5.
6. Holden BA, et al. Effects of long-term extended contact lens wear on the human cornea. Investigative Ophthalmology & Visual Science 1985;26:1489-501.
7. Covey M, et al. Hypoxic effects on the anterior eye of high-Dk soft contact lens wearers are negligible. Optometry and Vision Science 2001;78:95-9.
8. Sweeney DF. Have silicone hydrogel lenses eliminated hypoxia? Eye & Contact Lens 2013;39:53-60.
9. Nichols JJ. Contact lenses 2012. Contact Lens Spectrum 2013: January: 24-9.
10. Efron N, et al. Oxygen permeability and water content of silicone hydrogel contact lens materials. Optometry and Vision Science 2007;84:328-37.
11. Benjamin WJ. Oxygen Permeability and Transmissibility, Part 1. Contact Lens Spectrum 2008; February.
12. Kaufman PL, Alm KA. Adler’s Physiology of the Eye, Third Edition. St. Louis, MO: Mosby; 2003:92-3.
13. Riley MV. Glucose and Oxygen Utilization by Rabbit Cornea. Exp Eye Res 1969;8:193-&.
14. Brennan NA. Beyond flux: total corneal oxygen consumption as an index of corneal oxygenation during contact lens wear. Optometry and Vision Science 2005;82:467-72.
15. Brennan NA. Corneal oxygenation during contact lens wear: comparison of diffusion and EOP-based flux models. Clinical & experimental optometry : journal of the Australian Optometrical Association 2005;88:103-8.
16. Hill R. Oxygen uptake of the cornea following contact lens removal. J Am Optom Assoc 1965;36:913-5.
17. Holden BA, Mertz GW. Critical oxygen levels to avoid corneal edema for daily and extended wear contact lenses. Investigative Ophthalmology & Visual Science 1984;25:1161-7.
18. Harvitt DM, Bonanno JA. Re-evaluation of the oxygen diffusion model for predicting minimum contact lens Dk/t values needed to avoid corneal anoxia. Optometry and Vision Science 1999;76:712-9.
19. Papas E. On the relationship between soft contact lens oxygen transmissibility and induced limbal hyperaemia. Exp Eye Res 1998;67:125-31.
20. Morgan PB, et al. Central and peripheral oxygen transmissibility thresholds to avoid corneal swelling. Journal of biomedical materials research Part B, Applied biomaterials 2010;92:361-5.
21. Benjamin W. Defining hypertransmissibility of a contact lens. Contact Lens Spectrum 2008; August.
22. Eiden BS, DeNaeyer G. Keratoconus fitting with spectialty soft lenses. Contact Lens Spectrum 2012; January: 34-7.
23. Shovlin J. Softer approach to keratoconus? Review of Optometry 2012.
24. O’Donnell C, Codina CM. A hyper-Dk contact lens system for keratoconus. Eye Contact Lens 2004;30:44–8.
25. Romero-Jimenez M, et al. Which soft contact lens power is better for piggyback fitting in keratoconus? Contact Lens & Anterior Eye; 2013;36:45-48.
26. Michaud L, et al. Predicting estimates of oxygen transmissibility for scleral lenses. Contact Lens & Anterior Eye 2012;35:266-71.
27. Benjamin WJ. Oxygen transport through contact lenses. In: Guillon M, Ruben M., editors. Contact lens practice. Chapman Hall Medical Publishers; 1994: p 47-69.
28. Stapleton F, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 2008;115:1655-62.
29. Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low dk hydrogel extended contact lens wear. Optom Vis Sci 2007;84:247-56.
30. Chalmers RL, et al. Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the CLAY study. Invest Ophthalmol Vis Sci 2011;52:6690-6.
31. Chalmers RL, et al. Risk factors for contact lens complications in US clinical practices. Optom Vis Sci 2010;87:725-35.
32. Radford CF, et al. Risk factors for nonulcerative contact lens complications in an ophthalmic accident and emergency dept. Ophthalmology 2009;116:385-92.
33. Chalmers RL, et al. Multicenter case-control study of the role of lens materials and care products on the development of corneal infiltrates. Optom Vis Sci 2012;89:316-25.
34. Benjamin WJ, Karkkainen TR. Hydrogel hypoxia. Contact Lens Spectrum 1996; 11 (suppl):s6-11.


