 |
Even as we recognize that the newest contact lens development efforts give us viable options for everyone, including our most difficult patients, it is important to remember most lenses prescribed today in North America continue to be two-week and monthly disposables, despite the influx of daily replacement options. How, as eye care practitioners, can we help patients choose the right solution, and how does this help our goal of practice building? Reminding patients—both old and new—of proper contact lens care and solution use is key to maintaining a healthy patient population and even drawing in new ones.
A tremendous amount of research documents widespread lack of contact lens compliance, which can significantly affect the patient’s wearing experience.1-3 Although poor hygiene during lens care and handling typically dominates discussions of noncompliance, inattention to lens/solution compatibility when purchasing care products is also a concern among practitioners.
Patients face the challenge of selecting the right solution for their lenses following the initial fitting appointment, especially as many multipurpose solutions created decades ago remain packaged similarly to even the most recently engineered products. Intentional mimicry in product design by generic or store-brand products can mislead patients into making poor choices at the drugstore.
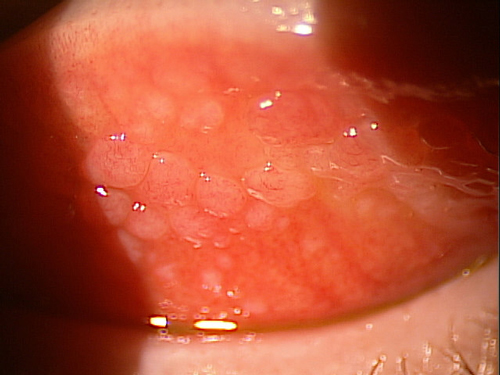 | |
| Fig. 1. Patients with giant papillary conjunctivitis may benefit from switching to a peroxide system. |
Know Your Solutions
Biotrue (Bausch + Lomb), OptiFree PureMoist (Alcon) and Revitalens OcuTec (Abbot Medical Optics) are three of the latest additions to the multipurpose solution options. Biotrue contains hyaluronic acid, a glycosaminoglycan that temporarily binds to the surface of the lens to increase daily comfort.5 OptiFree PureMoist contains the copolymer polyoxyethylene-polyoxybutylene under the trade name HydraGlyde, which is said to improve lens surface wettability and end-of-day comfort.6,7 Revitalens OcuTec contains the wetting agent Tetronic 904 in attempts to optimize surface moisture.8
Other disinfection options come in the form of hydrogen peroxide systems—most notably ClearCare (Alcon), the newer ClearCare Plus (Alcon) and PerioxiClear (Bausch + Lomb). Overall, peroxide systems have remained the mainstay for individuals with multipurpose solution sensitivities due to the lack of chemical preservatives in the peroxide. For those individuals prone to developing giant papillary conjunctivitis (GPC) who may not be good candidates for daily disposable contact lenses, hydrogen peroxide disinfection works remarkably well as a solution option after the patient’s GPC has been appropriately managed (Figure 1). And in fact, peroxide systems are having a bit of a renaissance lately, as practitioners see them as a possible bulwark against noncompliance. Patients can’t “top off” their solution if using peroxide, for instance.
ClearCare is a one-step peroxide system that combines disinfection and storage capabilities into a single entity. Patients can either rub and rinse the lenses with this solution and then place them in the lens case cage prior to submerging them in the solution, or place the lenses in the cage, rinse them for five seconds and then submerge the entire entity. An overnight soak in ClearCare requires six hours of neutralization prior to lens wear. Alcon recently added HydraGlyde to its ClearCare formulation under the name ClearCare Plus.
PeroxiClear, another one-step peroxide disinfection solution, has a four-hour soak requirement for neutralization. This time frame may be better suited for contact lens wearers with less consistent schedules who may require a faster cleaning method. Disinfection occurs via a platinum-modulating compound known as carbamide: when the platinum disc is submerged in peroxide, the carbamide binds to the platinum to inhibit the neutralization rate. This keeps the peroxide at a higher concentration than normal (i.e., when not in the presence of carbamide), and increases the total exposure of the lenses to the peroxide.9 During the first 60 minutes of soaking, the carbamide loses its affinity for the platinum disc, causing the platinum disc to rapidly neutralize the peroxide in the system. This rapid neutralization is what allows wearers to remove the lens from the peroxide solution after only a four-hour soak and comfortably place it on the eye.
Understand Their Differences
New technologies can help create better wearing experiences for the patient—which is why practitioners must remain cognizant of the number of store-brand peroxide systems that are also available. While similar at first to some of the branded systems, they, in fact, have significant differences that can irrevocably alter a patient’s wearing experience.
A number of structural differences exist between branded peroxide basket-and-disc systems and generic formulations. In non-branded peroxide solutions, the platinum disc is typically located at the bottom of the vial that holds the peroxide rather than attached to the basket that contains the lenses. While a seemingly trivial detail, this difference can affect the neutralization profile of the peroxide: because the platinum disc is attached to the basket containing the lenses, both the lenses and platinum disc are submerged into the peroxide simultaneously to provide maximum exposure of the lenses to the peroxide. In contrast, the placement of the platinum disc at the bottom of the vial in store-brand products means that the neutralization process begins as soon as the peroxide is placed in the vial. If a lens wearer delays placement of their lenses into the basket, the lenses’ total exposure time to peroxide is decreased—effectively lowering disinfection efficacy. Should the patient insist on using private label peroxide systems, let them know that that the lenses and lens basket should be submerged into the peroxide immediately after the peroxide has been placed in the vial containing the platinum disc.
Giving a little extra attention to contact lens care guidance means healthier, and thus happier, patients—and happier patients means more referrals.
1. Tilia D, Lazon de la Jara P, Zhu H, et al. The effect of compliance on contact lens case contamination. Optom Vis Sci. 2014 Mar;91(3):262-71.
2. Kuzman T, Kutija MB, Juri J, et al. Lens wearers non-compliance - is there an association with lens case contamination? Cont Lens Anterior Eye. 2014 Apr;37(2):99-105.
3. Dumbleton KA, Woods CA, Jones LW, Fonn D. The relationship between compliance with lens replacement and contact lens-related problems in silicone hydrogel wearers. Cont Lens Anterior Eye. 2011 Oct;34(5):216-22.
4. Morgan PB, Woods CA, Tranoudis IG, et al. International Contact Lens Prescribing in 2014. Contact Lens Spectrum. 2015 Jan;30:28-33.
5. Scheuer CA, Fridman KM, Barniak VL, et al. Retention of conditioning agent hyaluronan on hydrogel contact lenses. Cont Lens Anterior Eye. 2010 Dec;33 Suppl 1:S2-6.
6. Senchyna M, Stauffer P, Davis J, et al. Characterization of a multi-purpose lens solution designed for silicone hydrogel materials. IOVS. 2010 Apr;51(13):3426.
7. Napier L, Garofalo R, Lemp J, et al. Clinical evaluation of an investigational multi-purpose disinfecting solution. Poster presented at: Contact Lens Association of Ophthalmologists meeting, Sept. 2010, Las Vegas.
8. González-Méijome JM, da Silva AC, Faria-Ribeiro M, et al. Multi-site clinical assessment of Complete Revitalens MPDS in 2981 contact lens wearers across Europe and USA. Cont Lens Anterior Eye. 2013 Dec;36(6):289-93.
9. Millard KA, Hook D, Hoteling A, Wygladacz K. A one-step hydrogen peroxide-based contact lens solution. Contact Lens Spectrum. Special Edition 2014.
10. Contact Lens Research Services. Andrasko Corneal Staining Grid. Available at: www.staininggrid.com. Accessed November 20, 2015.


