 |
The approval of Rhopressa (netarsudil ophthalmic solution 0.02%, Aerie Pharmaceuticals) provides practitioners with the first novel agent for the treatment of intraocular pressure (IOP) for open-angle glaucoma and ocular hypertension since the approval of the prostaglandin analogue latanoprost in 2003. This combination Rho-kinase (ROCK) inhibitor is an exciting development for those who manage glaucoma because it will allow the addition of a second once-daily topical medication when there is inadequate control with a once-daily prostaglandin. In the past, practitioners would often need to add a medication dosed two or three times a day. This is beneficial from the standpoint of improved patient compliance, reduced cost and less issues with exacerbation of co-existing dry eye disease.1
In Rhopressa’s Phase III FDA clinical trials, dubbed Rocket 1, 2, 3 and 4, the efficacy of netarsudil 0.02% QD or BID was compared with timolol 0.5% BID. The results showed great potential for glaucoma patients, but not without some corneal concerns.2
The Trouble
In both Rocket 1 and 2, more subjects in the Rhopressa group discontinued use due to adverse events than those using timolol.2 One of the more common adverse events was conjunctival hyperemia. This is an expected pharmacological effect, considering ROCK inhibition is known to cause vasodilation, and the medication is preserved with benzalkonium chloride 0.015%. While patients did not report noticing an increase in redness, researchers noted some worsening of hyperemia in their slit lamp findings. If the redness is especially problematic for the patient, the medication can be discontinued and substituted with something that contains a different preservative or a preservative-free formulation.
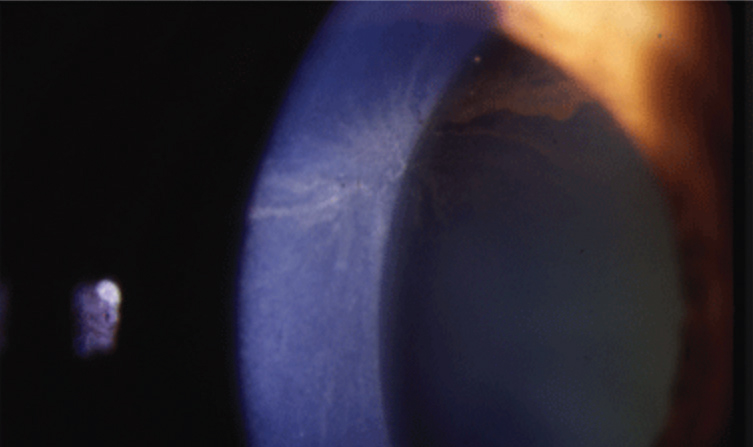 |
| Fig. 1. Corneal verticillata may be a common side effect of Rhopressa. Photo: Jay S. Pepose, MD, PhD |
Another more notable event was the incidence of corneal verticillata, or vortex keratopathy, which was present in about 21% of patients given once-a-day Rhopressa at the one-month mark in the four trials (Figure 1).2 These brownish/grayish subepithelial corneal deposits radiate in the central cornea in a “whorl” pattern, and are a notable side effect in patients taking a number of medications such as amiodarone, an antiarrythmic drug. In once-a-day Rhopressa patients, corneal verticillata was noted as similar in appearance to that associated with amiodarone, though milder and less distinct.
A follow-up study of 45 subjects who had ongoing corneal verticillata showed resolution with discontinuation in all but three subjects.2 After study completion, it was resolved in one of the remaining subjects and improved in the other two with no meaningful changes in visual function. So, while corneal verticillata appears to be a common side effect of this drug, it also appears to cause no degradation in vision and resolves with discontinuation.
About 17% of subjects in Rocket 1 and 2 also showed mild subconjunctival hemorrhages, which can be striking in appearance and can cause concern in uninformed patients. Prior to starting the medication, clinicians should educate patients that these hemorrhages can occur, and if they do, the “blood” in the eye is benign and will quickly resolve.
Additionally, a study that compared netarsudil with latanoprost found the most frequently reported adverse event was conjunctival or ocular hyperemia, which was noted in 24% of netarsudil subjects vs. 11% in latanoprost subjects (though this was generally mild and transient).3
In another netarsudil vs. latanoprost study, researchers found a greater incidence of mild, diminishing conjunctival hyperemia (40% in netarsudil patients vs. 14% in latanoprost patients).4
When prescribing Rhopressa for your open-angle glaucoma and ocular hypertension patients, keep in mind that a significant amount of them may experience mild, transient conjunctival hyperemia, and almost one quarter of patients may manifest corneal verticillata.
1. Chaglasian EL, Than T. Juggling Dry Eye and Glaucoma. RCCL. 2017;154(5):10-1. |


