Anterior segment ophthalmic imaging equipment—such as corneal topographers and anterior segment tomographers—can generate clear and detailed images of the corneas. Whereas corneal topographers measure 2-D images of the anterior cornea, anterior segment tomographers such as from slit scanning and optical coherence tomography (OCT), create 3-D images from two dimensional cross sections of the anterior segment. The different types of equipment offer powerful, non-invasive imaging, augmenting traditional slit lamp evaluations for the treatment and monitoring of corneal disease.
New Technology: Corneal Topography

1. Cross section of the anterior segment of a healthy eye taken with the Pentacam utilizing Scheimpflug imaging.
The latest corneal topographers have greatly advanced since their inception. Not only are they faster and more compact, but also more robust and more affordable—making them available for routine patients and not just those with corneal issues.
Corneal topographers analyze the anterior surface of the cornea primarily with Placido’s disks. The Placido’s disks’ concentric rings are reflected off the cornea and captured by a digital camera; the deviation of the reflected rings is then processed by software to calculate the shape of the cornea. However, in order to collect a clear image, an intact epithelial surface and tear film is required. Other disadvantages of corneal topographers include artificially induced astigmatism based off alignment and fixation errors, decreased accuracy of the peripheral corneal measurements and non-standardized algorithms between various brands of corneal topographers.
Because the topography programs assume a prolate cornea, non-prolate corneas or irregular corneal surfaces are often misdiagnosed and categorized simply as “irregular.” Nevertheless, indexes based on mathematical algorithms have been developed to assist in the diagnosis of corneal conditions, such as keratoconus and pellucid. However, it is still difficult to differentiate conditions, such as corneal warpage from contact lens wear versus keratoconus, without a thorough history and other clinical tests.
Corneal topography is instrumental for detecting and monitoring the progression of many entities and treatments including orthokeratology, keratoconus and lenticular astigmatism, as well as analyzing dry eyes.1-10
Independent studies have reported cases of progressive lenticular astigmatism as monitored by corneal topography.7,8 Repeated corneal topography over time showed no changes in the corneal curvature, hence confirming the diagnosis of progressive astigmatism by lenticular changes as opposed to corneal ectasia. After the astigmatism stabilized, patients underwent phacoemulsification and the corneal topographies were compared again. The lack of change in refractive error, as well as corneal topography, indicated that the progressive astigmatism had stabilized.
Corneal topographers also have been used to non-invasively measure and analyze the kinetics of dry eyes.9-11 Because tear film stability is needed to produce clear images, the irregular images caused by a poor tear film are captured in a series of images with the corneal topographer over a period of 10 seconds. The images are analyzed and quantified with a tear stability analysis software program to objectively measure the stability of tear film on the eye.
Corneal Tomography and Slit Scan Imaging
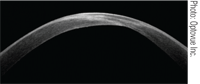
2. A high resolution, cross-sectional image of a keratoconic cornea taken with the RTVue, a spectral domain OCT.
Whereas corneal topographers are the more common and faster method of measuring the anterior segment of the cornea, corneal tomographers can create 3-D images, which provide more information than using the Placido’s disks alone. Slit scan imaging corneal tomographers include equipment that uses only slit scanning, such as the Orbscan (Technolas), and equipment that combines Scheimpflug imaging, such as the Pentacam (Oculus).
• Slit Scanning: Orbscan corneal tomographers (Technolas) use slit scanning to obtain images of the eye. Similar to a slit lamp’s light slits, optical slits are scanned along multiple points across the cornea. The reflections of the slits are captured by a video camera positioned at a 45º-angle from the cornea, which allows for measurements of both the anterior and posterior surfaces of the cornea, corneal pachymetry and the anterior surface of the lens and iris.
The limitations of these instruments include requiring a clear cornea and steady eye fixation because of the longer procedure time compared to Placido’s disk-based corneal topographers. Newer corneal tomographers use a combination of both Placido’s disk reflections and scanning slits to analyze the cornea.
• Scheimpflug Imaging: Pentacam (Oculus) is an example of a corneal tomographer using Scheimpflug imaging. It has a rotating Scheimpflug camera to capture cross-sectional slits of the anterior segment—allowing for a three-dimensional image of the anterior segment. The Scheimpflug camera images objects when their plane is not parallel to the film of the camera. The high resolution images can provide anterior and posterior corneal topography, pachymetry, anterior chamber depth, lens opacification and lens thickness.
The advantage of these corneal tomographers over Placido imaging alone includes the ability to more accurately measure corneas with severe irregularities and to calculate pachymetry from limbus to limbus. The limitations of these corneal tomographers include requiring steady eye fixation because of the longer time needed to obtain measurements. These corneal tomographers also have difficulties identifying changes in corneal interfaces, such as those with LASIK flaps, and may estimate a thinner cornea in corneas with corneal opacities or ectasia.
Currently available Scheimpflug imaging tomographers use combinations of rotating Scheimpflug cameras, stationary cameras and Placido’s disk reflections to obtain images of the anterior segment.
Studies have shown the efficacy of using slit imaging and Scheimpflug imaging tomographers to differentiate keratoconic eyes from normal eyes.12-16 Keratoconus indices have been made using videokeratography from Placido’s disk corneal topographers, but Scheimpflug imaging tomographer measurements may be more accurate, identifying 88% of contralateral eyes previously identified as unilateral keratoconus using standard criteria.5,12

3. Visante, an anterior segment
time-domain OCT device.
By being able to evaluate the posterior corneal elevation, pachymetry and refractive power changes with one device, slit scanning and Scheimpflug imaging tomographers are effective means for diagnosing and monitoring different stages of keratoconus and other corneal ectasia.12-14,17,18
Anterior Segment OCT
Similar to an ultrasound, but reflecting light instead of sound, anterior segment optical coherence tomography (OCT) non-invasively captures high-resolution cross-sections of the eye by assessing all the light waves that are reflected and scattered from the ocular structures. Low coherence interferometry analyzes the delay and intensity of the reflected light beams and compares it to light that has traveled along a referenced path. The axial scans (A-scans) are then combined to create a 3-D image, allowing for cross-sectional views of the cornea and anterior chamber.
The cellular-level images provide more in-depth evaluations of the cornea, anterior chamber and corneal thickness, and so allow for diagnosis of corneal abnormalities as well as tear film dynamics. There is no coupling medium and OCTs are non-contact, allowing for views of ocular structures in their natural state.
• Time Domain (TD) OCT: TD-OCTs are older generation tomographers that use a mechanical arm with a reference mirror that moves across the cornea. While varying the position of the reference mirror, A-scans are created serially (i.e., time domain). Due to the mechanical limitation of the reference mirror, there is a lag in scanning time of 2,000 axial scans per second. A-scans must also be well aligned to produce clear images, therefore it is more sensitive to motion errors.
Although TD-OCTs are slower than spectral domain (SD) OCTs, their lower wavelength can penetrate deeper into the eye. They can penetrate as much as 18μm through tissues such as the sclera, iris and opacified corneas, which makes them valuable instruments for anterior segment care.
In one instance, a TD-OCT was instrumental in the diagnosis and monitoring of the healing process of a case of fungal keratitis by identifying the infiltrate beneath an edematous cornea.19 Joseph Colin, M.D., and colleagues used a TD-OCT to measure the depth of granular corneal dystrophy opacities in one patient to help determine whether a deep anterior lamellar keratoplasty or a phototherapeutic keratectomy was needed to improve the vision of the affected eye.20 Similarly, TD-OCT was used to determine the depth of a corneal opacity, which then allowed for its accurate removal using phototherapeutic keratectomy.21
Other uses of TD-OCT include gas-permeable contact lens fitting and tear film assessments. Lugina Sobara, O.D., and collegues found that TD-OCTs can help with fitting large diameter gas-permeable contact lenses because they can effectively measure curvatures of the peripheral cornea and sclera, which are generally outside of the area normally viewed by corneal topographers.22 Shizuka Koh, M.D., Ph.D., and colleagues used a TD-OCT and wavefront sensor to quantify tear meniscus dimensions and optical quality to quantify dry eyes.23
• Spectral Domain (SD) or Fourier Domain (FD) OCT: Fourier domain or SD-OCTs are newer tomographers that are capable of producing higher resolution images. Instead of using a mechanically sweeping arm with a mirror as in TD-OCT, the reference light is fixed and the reflections generate spectral interference fringes that are captured through a Fourier domain spectrometer and a high resolution charged-coupled device camera. The signals are captured in parallel rather than serially and calculated electronically instead of mechanically allowing for faster imaging speeds up to 26,000 axial scans per second. The speed of image acquisition is limited to the camera’s frame capture rate and the computer speed of the Fourier calculation. The reduced examination time and the SD-OCT’s ability to track eye movement minimize errors from eye motion and improve repeatability of results.
Both SD-OCTs and TD-OCTs are reliable tomographers and can be used for similar evaluations.24,25 SD-OCT’s higher resolution imaging, however, improves sensitivity and repeatability of measured results. A custom built, ultra high-resolution SD-OCT can also be sufficient to study epithelial, Bowman’s layer or endothelial conditions up to 2µm.26
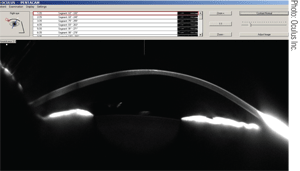
4. A cross-sectional image of a keratoconic cornea taken with the Pentacam.
Similar to TD-OCTs, SD-OCTs have been used to measure depths of corneal opacities from scars and dystrophies to help determine appropriate treatment plans. Lejla Vajzovic, M.D., and colleagues obtained histological profiles with their SD-OCT to treat corneal dystrophy of Bowman’s layer, spheroidal degeneration, Salzmann’s nodular degeneration, granular dystrophy, macular dystrophy, anterior basement membrane dystrophy, Meesman’s dystrophy and ocular surface squamous neoplasia.26 Tara A. McCannel, M.D., and colleagues documented the treatment and resolution of a conjunctival mucosa-associated lymphoid tissue.27
SD-OCTs have been used to monitor the progression of epithelial healing under therapeutic contact lenses after lamellar keratoplasty and epi-LASIK procedures.28 Others have used SD-OCTs to access intralcorneal stromal ring insertions in patients with keratoconus.29,30 SD-OCTs have also been used to measure dry eyes by quantifying tear meniscuses.31,32 Cross-sectional images of the tear meniscus with the SD-OCT are magnified to more accurately estimate the volume of tears in dry eye and normal patients.
There are many types of anterior segment imaging equipment to choose from, and the wealth of information gathered from these devices can make their clinical applications seem almost limitless. Depending upon the clinical needs of the office, corneal topographers and tomographers augment clinical biomicroscopic evaluations to not only better diagnose and manage patients’ anterior segment entities, but also quantitatively monitor their progress over time.
Dr. Yeung, O.D., is the director of the UCLA Arthur Ashe Student Health Optometry Clinic. She is a diplomate in Cornea, Contact Lenses and Refractive Technologies through the American Academy of Optometry.
Steven Chang is a 3rd year UCLA undergraduate pre-optometry student majoring in Physiological Sciences.
1. Kojima T, Hasegawa A, Hara S, et al. Quantitative evaluation of night vision and correlation of refractive and topographical parameters with glare after orthokeratology. Graefes Arch Clin Exp Ophthalmol. 2011 May 12. [epub ahead of print]
2. Hiraoka T, Mihashi T, Okamoto C, et al. Influence of induced decentered orthokeratology lens on ocular higher-order wavefront aberrations and contrast sensitivity function. J Cataract Refract Surg. 2009 Nov;35(11):1918-26.
3. Mathur A, Atchison DA. Effect of orthokeratology on peripheral aberrations of the eye. Optom Vis Sci. 2009 May;86(5):E476-84.
4. Gatinel D, Malet J, Hoang-Xuan T, Azar DT. Corneal elevation topography: best fit sphere, elevation distance, asphericity, toricity, and clinical implications. Cornea. 2011 May;30(5):508-15.
5. Rabinowitz YS. Videokeratographic indices to aid in screening for keratoconus. J Refract Surg. 1995 Sep-Oct;11(5):371-9.
6. Li X, Yang H, Rabinowitz YS. Keratoconus: classification scheme based on videokeratography and clinical signs. J Cataract Refract Surg. 2009 Sep;35(9):1597-603.
7. Tatham A, Prydal J. Progressive lenticular astigmatism in the clear lens. J Cataract Refract Surg. 2008 Mar;34(3):514-6.
8. Tint NL, Jayaswal R, Masood I, Maharajan VS. Rapidly progressive idiopathic lenticular astigmatism. J Cataract Refract Surg. 2007 Feb;33(2):333-5.
9. Goto T, Zheng X, Klyce SD, et al. A new method for tear film stability analysis using videokeratography. Am J Ophthalmol. 2003 May;135(5):607-12.
10. Kojima T, Ishida R, Dogru M, et al. A new noninvasive tear stability analysis system for the assessment of dry eyes. Invest Ophthal Vis Sci. 2004 May;45(5):1369-74.
11. Németh J, Erdélyi B, Csákány B, et al. High-speed videotopographic measurement of tear film build up time. Invest Ophthalmol Vis Sci. 2002 Jun;43(6):1783-90.
12. Uçakhan ÖÖ, Cetinkor V, Özkan M, Kanpolat A. Evaluation of Scheimpflug imaging parameters in subclinical keratoconus, keratoconus, and normal eyes. J Cataract Refract Surg. 2011 Jun;37(6):1116-24.
13. De Scanctis U, Loiascono C, Richiardi L, et al. Sensitivity and specificity of posterior corneal elevation measured by Pentacam in discriminating keratocnus/subclinical keratoconus. Ophthalmology. 2008 Sep;115(9):1534-9.
14. Ambrósio R Jr, Alonso RS, Luz A, Coca Velarde LG. Corneal-thickness special profile and corneal volume distribution: tomographic indices to detect keratoconus. J Cataract Refract Surg. 2006 Nov;32(11):1851-9.
15. Kovács I, Mihâltz K, Ecsedy M, et al. The role of reference body selection in calculating posterior corneal elevation and prediction of keratoconus using rotating Scheimpflug camera. Acta Ophthalmol. 2011 May;89(3):e251-6.
16. Wei RH, Zhao SZ, Lim L, et al. Incidence and characteristics of unilateral keratoconus classified on corneal topography. J Refract Surg. 2011 May;10:1-7.
17. Fernandes M. Scanning slit topography: diagnostic boon in presumed unilateral Terrien’s marginal degeneration. CLAE. 2011 Jun 10. [epub ahead of print]
18. Núñez MX, Blanco C. Efficacy of Orbscan II and Pentacam topographers by a repeatability analysis when assessing elevation maps in candidates to refractive surgery. Biomedica. 2009 Sep;29(3):362-8.
19. Martone G, Pichierri P, Franceschini R, et al. In vivo confocal microscopy and anterior segment optical coherence tomography in a case of alternaria keratitis. Cornea. 2011 Apr;30(4):449-53.
20. Combillet F, Touboul D, Leger F, Colin J. Granular corneal dystrophy treated with deep anterior lamellar keratoplasty: comparing histological analysis and optical coherence tomography. J Fr Ophthalmol. 2011 Jun 14. [epub ahead of print]
21. Ma JJ, Tsent SS, Yarascavitch BA. Anterior segment optical coherence tomography for transepithelial phototherapeutic keratectomy in central corneal stromal scarring. Cornea. 2009 Sep;28(8):927-9.
22. Sobara L, Maram J, Fonn D, et al. Metrics of the normal cornea: anterior segment imaging with the Visante OCT. Clin Exp Optom. 2010 May;93(3):150-6.
23. Koh S, Tung C, Aquavella J, et al. Simultaneous measurement of tear film dynamics using wavefront sensor and optical coherence tomography. Invest Ophthalmol Vis Sci. 2010 Jul;51(7):3441-8.s
24. Wylegala E, Teper S, Nowiñska A, et al. Anterior segment imaging: Fourier-domain optical coherence tomography versus time-domain optical coherence tomography. J Cataract Refract Surg. 2009 Aug;35(8):1410-4.
25. Li H, Leung CK, Wong L, et al. Comparative study of central corneal thickness measurement with slit-lamp optical coherence tomography and Visante optical coherence tomography. Ophthalmology. 2008 May;115(5):796–801.
26. Vajzovic L, Karp C, Haft P, et al. Ultra high-resolution anterior segment optical coherence tomography in the evaluation of anterior corneal dystrophies and degenerations. Ophthalmol. 2011 Jul;118(7):1291-6.
27. Williams BK Jr, Tsui I, McCannel TA. Spectral-domain optical coherence tomography of conjunctival mucosa-associated lymphoid tissue lymphoma with presumed choroidal involvement. Graefes Arch Clin Exp Ophthamol. 2010 Dec;248(12):1837-40.
28. Pang CE, Vanathi M, Tan D, Mehta J. Evaluation of corneal epithelial healing under contact lens with spectral domain anterior segment optical coherence tomography. Open Ophthalmol J. 2011 Jun;5:51-4.
29. Lai MM, Tang M, Andrade EMN et al. Optical coherence tomography to assess intrastromal corneal ring segment depth in keratoconic eyes. J Cataract Refract Surg. 2006 Nov;32(11):1860-5.
30. Rapuano CJ, Sugar A, Koch DD, et al. Intrastromal corneal ring segments for low myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2001 Oct;108(10):1922-8.
31. Park DI, Lew H, Lee SY. Tear meniscus measurement in nasolacrimal duct obstruction patients with Fourier-domain optical coherence tomography: novel three-point capture method. Acta Opthalmol. 2011 July 5. [epub ahead of print]
32. Gumus K, Crockett CH, Pflugfelder SC. Anterior segment Optical Coherence tomography: A diagnostic instrument for conjunctivochalasis. Am J Ophthalmol. 2010 Dec;150(6):798-806.


