Sjögren's syndrome has a bit of a Dr. Jekyll/Mr. Hyde duality in eye care. On the one hand, we are all well aware of the condition and understand its ophthalmic association. On the other, the prevalence of Sjögren’s is grossly underestimated and infrequently mentioned to patients. It is estimated that Sjögren’s syndrome affects four million people in the United States alone, yet three million of those who suffer from this disease have not yet been diagnosed.1 Clinicians routinely fail to recognize and diagnose Sjögren’s patients at an early enough stage to have an impact on their quality of life.
As with many autoimmune diseases, the presentation of Sjögren’s syndrome can be highly variable. In some patients, Sjögren’s can present as a fairly aggressive, rapidly advancing, severe disease; in others, it can present in a relatively mild state. Because the presentation of Sjögren’s can vary so much between patients, it can easily be mistaken for typical dry eye or age-related dryness in its earlier stages.
We must start to discuss Sjögren’s syndrome—with our peers and our patients—to fully understand the disease and its systemic manifestations, as well as the most effective way of managing patients who suffer from it.
How to Spot Sjögren’s
When most of us think of Sjögren's syndrome, an archetypal patient readily comes to mind: the post-menopausal woman presenting with a classic aqueous-deficient dry eye. Beyond the characteristic findings of dry eye and often—but not always—dry mouth, Sjögren’s syndrome is a chronic and systemic disease with the potential to affect many body systems.
Lack of salivary production in the advanced stages of the disease leads to a number of additional complications, including dental decay, difficulty swallowing, bleeding cracks in the gums and severely chapped lips. The autoimmune component of Sjögren’s, which is more often the secondary form of the disease, has a deleterious effect on the organs.
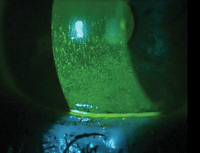 | |
|
Fluorescein staining revealing dry eye. Staining is a hallmark sign of
Sjögren’s syndrome and can be used to assist diagnosis of the disease in its early stages.
Photo: Mile Brujic, OD
|
For example, it can cause pneumonia, interstitial lung disease and recurrent bronchitis in the lungs; acid reflux, esophagitis and difficulty swallowing in the gastrointestinal system; and primary biliary cirrhosis as well as autoimmune hepatitis in the liver. There are also neurological symptoms, such as memory loss and “brain fog.”
Additionally, because Sjögren’s affects all the body’s mucous membranes, patients often experience recurrent sinusitis, nosebleeds, acid reflux, breathing problems, bronchitis, dry skin, Raynaud's phenomenon, abnormal liver function and peripheral neuropathy. All of these complications worsen quality of life, so it is important that we make the diagnosis early. We must realize that we are treating more than just the eyes of our Sjögren’s patients.
Diagnosis
Staining is a hallmark sign of Sjögren’s syndrome. In a 2010 study, researchers compared 231 patients with primary Sjögren's syndrome to 89 patients with aqueous-deficient dry eye to determine the objective signs that best differentiated the two conditions. They found that rose bengal staining of the temporal conjunctiva was the most important variable that separated the two groups. This staining and the severity of dry mouth symptoms were the major factors in distinguishing Sjögren’s syndrome patients from those with aqueous-deficient dry eye.2
Another telltale sign of Sjögren’s syndrome is an elevated tear osmolarity score. Typically, patients with Sjögren’s exhibit scores of 330 and above—with some variance between the two eyes. Due to the absence of reflex tearing, non-anaesthetized Schirmer's testing tends to be an accurate diagnostic measure in this group. This is unusual because Schirmer's testing is often inaccurate in the general dry eye population.
The disease is most likely to initiate in women in their 30s and 40s. Additionally, it is typically more prevalent in patients with an existing autoimmune disease, such as lupus or rheumatoid arthritis. While there is no cure for Sjögren’s, the morbidity of the disease can be lessened thanks to new therapies.
Most patients are diagnosed with Sjögren’s syndrome late in its course. Unfortunately, due to the progressive nature of the disease, it is significantly more difficult to manage at that point. However, the future of diagnosing Sjögren’s looks bright. As technology continues to advance, new options for the early diagnosis of Sjögren’s are becoming available.
For example, an in-office panel test for early detection of Sjögren’s (Sjö, Nicox) was recently launched in the United States. The test combines traditional Sjögren’s biomarkers (e.g., SSA (Ro), SSB (La), anti-nuclear antibody and rheumatoid factor) with additional biomarkers (e.g., salivary protein 1, carbonic anhydrase 6 and parotid secretory protein) that have also been associated with Sjögren’s at an earlier stage of disease progression. The eye care professional takes blood samples (where allowed) from potential Sjögren’s patients in the clinic and receives accurate lab results in days.
Sjögren’s often accompanies other autoimmune diseases. As such, when a patient presents with complaints of dry eye, as well as an autoimmune disease such as lupus or rheumatoid arthritis, it is important to consider Sjögren’s immediately. While there is a possible association between rheumatoid arthritis and dry eye, it is important to be mindful that a significant number of these patients may have Sjögren’s syndrome in addition to an autoimmune disease.3 We must find ways to differentiate between the two.
Thoughts on Managing Sjögren’s Syndrome
Due to a lack of available topical options for treating late-stage Sjögren’s patients, steroids are a mainstay for the condition. However, proceed with caution with the use of steroids if the patient has a poorly controlled autoimmune disease. If there's a systemic component that is not well controlled, such as arthritis, there is a risk of a corneal melt or secondary infection when using corticosteroids.
Oral secretagogues have been shown to be worthy alternative to steroids. These agents have a favorable safety profile, and research shows that cevimeline is safe and effective in improving symptoms of dry eye.4 Additionally, Sjögren’s patients respond well to preservative-free artificial tears because of the hyper-osmolarity associated with the condition—particularly artificial tears that can significantly lower hyper-osmolarity levels (e.g., TheraTears, Blink and FreshKote).5
Once a Sjögren’s syndrome patient is well controlled, he or she can often be maintained on topical cyclosporine (Restasis, Allergan). This must be done early, however, because once the scarring of the lacrimal gland occurs, Restasis will no longer be a viable option—Restasis can stimulate tear production and reduce inflammation, but it cannot repair or restore tissue that is destroyed. Nutritional supplements, such as HydroEye (Science Based Health), may also be useful for maintaining Sjögren's patients on a long-term basis.
A recent study demonstrated that GLA (e.g., black currant seed oil) in the presence of DHA/EPA (i.e., fish oil) appears to stimulate PGE1 significantly in the tears, which improved both signs (corneal staining) and symptoms (OSDI).6 Other options that have worked well include moisture chamber goggles, autologous serum, warm compresses, humidifiers, amniotic membrane rings, punctal occlusion and scleral lenses. Some success has been achieved using bandage contact lenses made of newer hydrophilic materials.
As is the case with treatment options for any condition, risks and benefits should be carefully weighed before making any decisions. Until advanced medications are developed for Sjögren’s patients, we must try our best to manage and control the ocular symptoms while involving other physicians to assist in controlling the systemic component of the disease.
Building Relationships
Optometry is increasingly the first point of contact for many systemic conditions, Sjögren’s syndrome included. If we diagnose Sjögren’s early, we can track the progression of the disease more precisely and work with primary care physicians and other specialists to ensure patients are followed, accruing benefits along the entire path of the disease course, and its care, will follow for the patient.
While optometrists commonly manage patients with Sjögren’s syndrome, ophthalmologists may be less interested in doing so—managing Sjögren’s patients can be very time consuming, and dry eye work-ups are rather extensive and sometimes unpleasant for the patient.
The average time it takes for a patient with Sjögren’s to receive a diagnosis is 4.7 years.7 The idea that these patients are floating around undiagnosed for almost five years is a significant cost to the health care system and to the morbidity these patient endure.
Optometrists and other health professionals tend to underestimate the potential for mutual cooperation. As such, communication is vital. The key to success is the patient’s rheumatologist controlling the systemic disease, as this will greatly help with the accompanying keratoconjunctivis sicca. Additionally, because of the high correlation between lymphoma and Sjögren’s, it is also important that the rheumatologist monitor for lymphoma. Because we are more likely to diagnose Sjögren’s in our severe dry eye patients, it is our responsibility to communicate this closely with rheumatologists.
When thinking of patients with Sjögren’s it is also important to consider the old adage, “when you hear hoof beats you think of horses—not zebras.” We too often think we are dealing with a case of typical dry eye before we consider Sjögren’s syndrome. This wastes valuable health care resources and, more importantly, can cause a patient to suffer unnecessarily.
Beginning discussions about Sjögren’s syndrome in the eye care community is an important and long overdue step in the right direction. By working closely with other health care providers, we will create opportunities to improve quality of life for our Sjögren’s patients in sustainable ways.
1. Sjögren’s Syndrome Foundation. 2001. Available at http://www.sjogrens.org. Accessed September 5, 2013.
2. Caffery B, Simpson T, Wang S, et al. Rose bengal staining of the temporal conjunctiva differentiates Sjögren's syndrome from keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2010;51(5):2381-7.
3. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren’s Syndrome. Arch Intern Med. 2004;164: 1275-1284.
4. Ono M, Takamura E, Shinozaki K, et al. Therapeutic effect of cevimeline on dry eye in patients with Sjögren's syndrome: a randomized, double-blind clinical study. Am J Ophthalmol. 2004;138(1):6-17.
5. Gilbard JP. Dry eye: pharmacological approaches, effects, and progress. CLAO J. 1996 Apr;22(2):141-5.
6. Aragona P, Bucolo C, Spinella R, Giuffrida S, Ferreri G. Systemic omega-6 essential fatty acid treatment and pge1 tear content in Sjögren's syndrome patients. Invest Ophthalmol Vis Sci. 2005 Dec;46(12):4474-9.
7. About Sjögren’s Syndrome. Sjögren’s Syndrome Foundation Web site. http://www.sjogrens.org. Accessed November 11, 2013.


