Addressing meibomian gland dysfunction, and blepharitis in general, is the key to suppressing the inflammatory nature of dry eye disease (DED). However, artificial tears (ATs) still play a pivotal role in managing the condition. They are particularly effective at providing symptomatic relief to patients, especially during flare-ups. Advise DED patients to use tears regularly “like a lip balm” and not wait until their ocular surface is compromised and symptomatic. DED can’t easily be categorized in a binary classification of evaporative or aqueous deficient: TFOS DEWS II found up to 70% of sufferers have a mix of the two. Aside from symptomatic relief, artificial tears can reduce inflammation and help prevent epithelial cell death. When chosen carefully, eye drops can play a significant role in the management of dryness.
The amount of eye drops available can make selection overwhelming for a doctor (let alone a patient). Let’s explore some favorites and clarify when they are most appropriate. We will also simplify when it makes sense to turn to biological drops in the management of DED.
Editor’s Note: Not all products discussed are available in the US.
 |
|
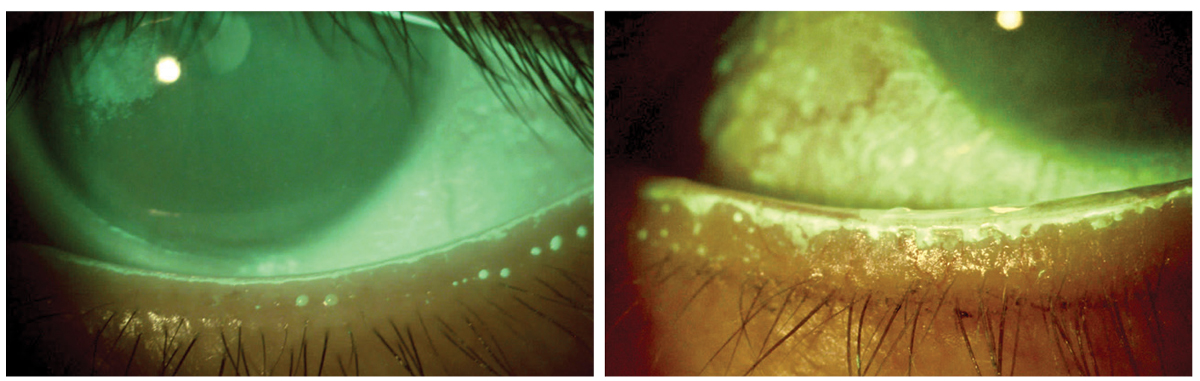
Figs. 1A and B. Normal line of Marx highlighted by fluorescein stain (left). Irregular line of Marx displaced posteriorly (away from the bulbar conjunctiva) in chronic MGD. All photos by Mahnia Madan, OD. Click image to enlarge. |
Dropping In
Preservatives in multi-dose bottles are considered a necessary evil to contain bacterial replication and minimize contamination risk. However, they are counterproductive: an irritant is being introduced to an already compromised tear film and ocular surface. Preservative-free formulations are always superior but should be highly recommended for those using drops more than four times a day. Benzalkonium chloride and thimerosal formulations should be avoided at all costs.
Tear osmolarity can be used as a guideline for selecting AT viscosity. Moderate to severe DED often necessitates a thicker drop. Generally speaking, as viscosity increases the duration of effect of the drop increases—but so does the potential for blurred vision.
A relatively inexpensive and effective option for mild to moderate DED is Systane Ultra Hydration (Alcon). It’s a moderately viscous drop that contains hyaluronate. Another ingredient, hydroxypropyl-guar (HP-Guar), interacts with the blinking motion to prolong on-eye contact time. HP-guar molecules bind preferentially to dried or compromised hydrophobic areas of the cornea, containing further damage while epithelial cells regenerate. It forms a gel layer (acting as a mucomimetic), compensating for a compromised tear layer and reducing friction during blinks.1
Systane Ultra also comes in a single dose non-preserved option, which is substantially more expensive but highly recommended if using drops more than four times a day.
A top-shelf multi-dose preservative-free option for more advanced dry eye is Hylo Dual Intense (Candorvison). It combines ectoine (a natural anti-allergy and anti-inflammatory agent) with a higher viscosity level (produced by a high concentration of heavier molecular weight sodium hyaluronate) that does not blur vision. Ectoine has been found effective in DED and allergic conjunctivitis. It has even been shown to accelerate wound healing post-op.2 The unique multi-dose pump does not allow air to penetrate the interior, keeping it safe for its six-month lifespan (once opened). When compared head-to-head with single-dose non-preserved options, this product’s cost becomes more defensible.
Thealoz Duo Gel (Labtician-Thea), a single unit preservative-free thicker gel, is an excellent bedtime option. It does not blur vision and is not oily. Trehalose (also found in Refresh Optive Mega 3) is an osmoprotectant designed to guard dried epithelial cells and stabilize their membranes. Simply put, trehalose protects against the destructive inflammatory cascade of DED. Sodium hyaluronate (as a glycosaminoglycan) enhances viscosity. Carbomer (a water-soluble polymeric resin) increases viscosity and maintains the hyaluronic acid and trehalose together in contact with the ocular surface for six hours without being sticky.3-5 The single-unit dose does make it a more expensive option among the nighttime alternatives.
A unique product for MGD is preservative-free Calmo spray (CandorVision). It is used with the eyes closed, which allows it to seep into the eye slowly, replicating meibomian gland secretions. It’s also an excellent option for people who hate putting drops into their eyes. The product contains liposomes to replicate the oil-deficient layer in MGD sufferers and dexpanthenol (pro-vitamin B5), which moisturizes the eye and surrounding skin.
Optase Hylo Night (Scope Health) is a nighttime ointment that uses vitamin A to speed up epithelial healing.6 It is preservative-free and good for mild to moderate dry eye. It is also phosphate free and good for six months once opened.
Refresh Lacri-Lube ointment (Allergan) is the go-to for very thick overnight coverage. It uses mineral oil as an ointment base that allows melting at body temperature and white petroleum as a lubricant.7 If inserting the ointment in both eyes, patients need to be warned that it will blur them out for a sustained period. Ideally, they should already be in bed when inserting it, for safety.
The Liposic (Bausch + Lomb) line has been a reasonably priced option for decades. While MGD patients don’t always respond to oil replenishment drops, this particular product has endured in both drop and ointment form (for nighttime use). The drops contain carbomer, sorbitol, medium-chain triglycerides and cetrimide preservative. Liposic gel has sodium hydroxide, which closely mirrors tear pH, and attempts to replicate all three tear layers.
Refresh Optive Mega-3 (Allergan) is a single-dose preservative-free drop. As the name suggests, it contains omega-3 from flaxseed oil. Studies show that eye drops using emollients can increase lipid layer thickness for a short duration.8 Omega-3 fatty acids are actually found in the normal tear film. Refresh Optive Mega-3 is formulated to minimize blur and does not require shaking. It is designed to replenish all three tear layers and is targeted towards MGD patients (like Systane complete and Retaine). Its lubricants include glycerin 1%, carboxymethylcellulose sodium 0.5% and polysorbate 80 (0.5%). These drops may be most helpful for patients with prolonged screen time, a lifestyle that decreases blinking and meibum secretion.8
There are many other excellent products on the market for DED. While there is no magic formula or perfect drop for every patient, a careful case history and an understanding (by both doctor and patient) that there will be some trial and error in finding the right products is key.
 |
|
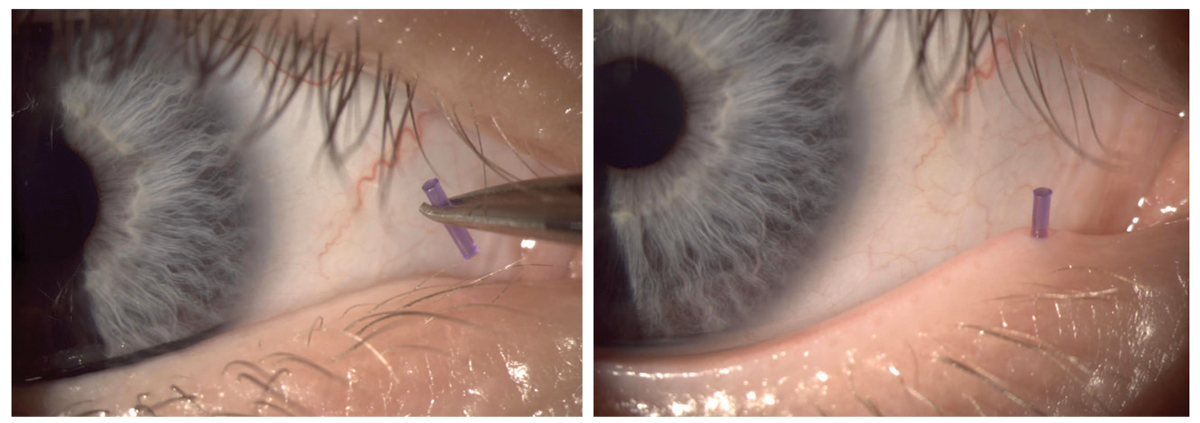
Fig. 2. Placing punctal plug in a lagophthalmos patient to increase tear volume. Click image to enlarge. |
When To Plug?
Punctal occlusion to preserve tear volume was a more popular option when we thought DED was effectively an absence of tears. We now know inflammation is the root cause. Recent years have seen a reduction in punctal occlusion—the thought is that occlusion keeps inflammatory mediators in the eye.9 However, the procedure should not be dismissed outright. It is still a key player in patients who have neurotrophic components, aqueous-deficient dry eye or non-resolving persistent epithelial defects (as in lagophthalmos patients). Increasing the tear volume can be extremely beneficial in these patients.10
Another benefit of punctal occlusion: it can increase the effectiveness of other medications used in the dry eye treatment, as it allows the drug to stay on the ocular surface longer. In one study, punctal plugs used with the cyclosporine group had longer symptom relief than either group treated separately.11
Before reaching for punctal plugs, though, manage ocular inflammation first with other methods. Punctal plugs are contraindicated when there are signs of active infection or allergies present (Figures 1a and 1b). Plugs are also not ideal in patients with blepharitis or meibomianitis, as they need management of their lid disease first.
Options range from dissolvable collagen inserts to more permanent silicone-type plugs. Temporary plugs can be used diagnostically to see if patients will benefit from longer plug use. Collagen plugs have a design advantage, too, as they lack the cap that is found in silicon plugs and can sometimes be irritating for patients (Figure 2).
 |
|
Fig. 3. View of autologous serum on left and platelet rich plasma on the right. Click image to enlarge. |
Typically, plugs are placed in the lower puncta; however, all four can be plugged if needed. Punctal plugs may not have a significant impact when used alone in the treatment algorithm, but when combined with other treatment modalities, patients are likely to benefit. Current manufacturers include: Odyssey, Katena, Lacrimedics and others.
In the not so far future, we will be seeing punctal plug-based drug delivery systems for glaucoma, allergies and even dry eye. These will address compliance issues and are more convenient for patients. They also have the potential to reduce ocular surface and systemic side effects of drugs.
Blood Biologics
For more severe dry eye sufferers, artificial lubricating drops are simply not enough. TFOS DEWS II reported that our natural tear film is a complex structure containing over 1,800 molecules that work together to not only form the most perfect lubricant, but the tear film also protects and nourishes the ocular surface. Our natural tears are epitheliotropic, which means they can support the proliferation, migration and differentiation of corneal and conjunctival cells.12 This is not something over-the-counter lubricating drops have been able to replicate.
When patients present with moderate to severe dry eyes with significant punctate keratitis, we can turn to blood biologics to help rescue and rehabilitate the ocular surface. The two common options are autologous serum eye drops (ASED) and platelet-rich plasma (PRP) eye drops.
Both have been successfully used in the treatment of moderate to severe DED; however, research suggests that PRP is superior in restoring the ocular surface.13 This is because PRP contains platelets, which are considered the powerhouses for healing. Platelets are the first cells to arrive at the wound site; they adhere to damaged tissue and initiate a healing reaction that includes the release of a variety of cytokines and growth factors. Similar to the natural tear film, growth factors released by platelets have epitheliotropic properties and are responsible for cell growth, collagen production, cell adhesion and healing of corneal and conjunctival cells, thus improving signs and symptoms of DED.13,14
Platelets are eliminated in the production of ASED, decreasing its potency. ASEDs are often diluted before dispensing, which further reduces the growth factors (Figure 3).
Both ASED and PRP drops are particularly helpful in recalcitrant dry eye (e.g., neuropathic and neurotrophic). PRP can help increase corneal nerve density lost in chronic DED.15 It is beneficial in multiple conditions, including recurrent corneal erosions, persistent epithelial defects, post-LASIK dry eye and Sjögren’s syndrome (Figure 4).13,14
Contraindications to ASED and PRP drops are few but barriers to availability are many. Both require regular blood draw and processing of blood, which may not be feasible for everyone. Typical blood draw yields a three-month supply of ASED or PRP eye drops, which are often used four to six times per day. They also require refrigeration. Once improvement in ocular surface disease is noted, frequency can be tapered and patients can be maintained on other therapies.
Optometrists can produce blood biologics in their practice (subject to state law) or work with local compounding pharmacies. Vital Tears (vitaltears.org) is another option for ODs looking to access ASED.
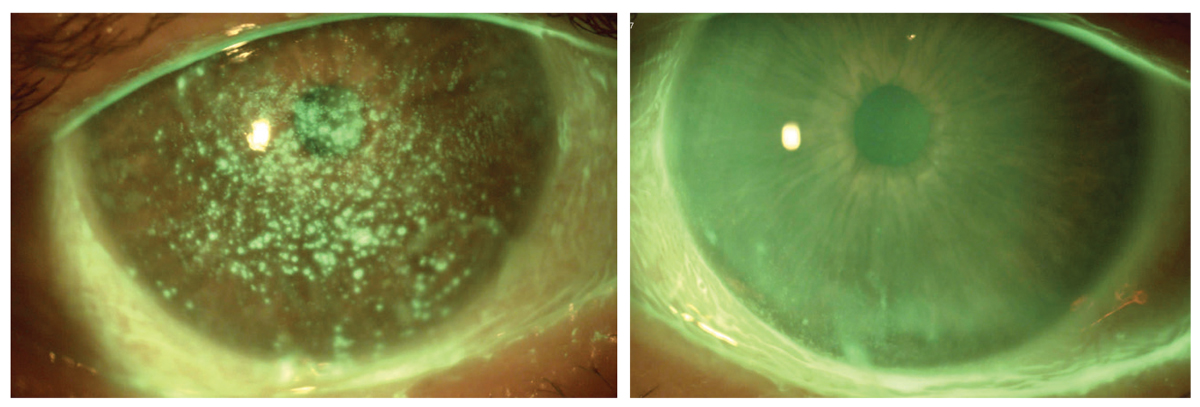 |
Fig. 4. Resolution of persistent epithelial defects with PRP and lid hygiene for three months. Click image to enlarge. |
Amniotic Fluid
If drawing the patient’s blood is not an option, practitioners can also consider biological eye drops derived from donor human amniotic fluid or placenta. These are indicated for mild to severe DED and can be a good option for patients looking for lubricants from natural sources. One study found that topical application of amniotic membrane extract eye drops reduced pain and inflammation and promoted re-epithelialization in ocular chemical burns.16
Options here include StimulEyes (M2 Biologics) and Regener-Eyes (Regener-Eyes) eye drops. Both are preservative-free and contain cytokines, chemokines and growth factors to aid ocular surface healing.17
Grow Some Nerve
When neurotrophic keratitis (NK) is suspected, cenegermin eye drops may also be used. Cenegermin is a recombinant nerve growth factor (rhNGF), produced in Escherichia coli, can promote corneal healing in a neurotrophic cornea.18,19 Oxervate (cenegermin-bkbj 0.002%, Dompé) is a sterile, preservative-free eye drop. It is available in seven multi-dose vials (1.0mL) intended to be used six times a day for eight weeks.
NGFs are known to regulate sensitivity in a normal cornea, which is important for epithelial healing. When persistent corneal staining or non healing epithelial defects are present and corneal sensitivity is reduced, neurotrophic keratitis should be suspected. Although rare, when it does occur NK is challenging to manage, as patients can develop non-healing corneal ulcers and even perforation due to the cornea’s inability to heal.20
In the current clinical studies, significant improvement in corneal healing was noted in the cengermin treatment group vs. the placebo group.21,22 However, whether there is improvement in corneal sensitivity in patients of the treatment groups is still debatable.18 Interestingly, use of bandage contact lenses along with cenergemin drops improved corneal sensation in 79% of the eyes in a recent retrospective study.23 Most common side effects of this therapy include hyperemia and eye pain, and it’s important to note that patients can relapse when drops are discontinued, suggesting the need for ongoing treatment and additional therapies.18
Just like with artificial tears, biologics and advanced alternatives are not a silver bullet. Every patient is best served through an individualized treatment plan that matches their experience in the DED spectrum. As eyecare providers, we must be well-informed on all options available to make that proper connection between a patient condition and appropriate care.
Dr. Madan practices in Vancouver, is president of the British Columbia Doctors of Optometry and has pioneered a novel technique to produce PRP eye drops in her clinic. She frequently speaks on treatments for advanced dry eye disease. She has advisory positions for Lumenis, Labtician and Sun Pharmaceuticals.
Dr. Eltis, based in Toronto, has presented and published worldwide and has been sought as a medical-legal consultant. He is a Fellow of the American Academy of Optomery, a Diplomate of the American Board of Optometry and a member of the Optometric Glaucoma Society. He has advisory positions for CooperVision, Volk, Heine and Sun Pharma.
1. Ng A, Keech A, Jones L. Tear osmolarity changes after use of hydroxypropyl-guar-based lubricating eye drops. Clin Ophthalmol. 2018;12:695-700. 2. Bilstein A, Heinrich A, Rybachuk A, Mösges R. Ectoine in the treatment of irritations and inflammations of the eye surface. Biomed Res Int. 2021;8885032. 3. Marner K, Møoller PM, Dillon M, Rask-Pedersen E. Viscous carbomer eye drops in patients with dry eyes. Efficacy and safety. a randomized, open, cross-over, multicenter study. Acta Ophthalmol Scand. 1996;74(3):249-52. 4. Sullivan LJ, McCurrach F, Lee S, et al. Efficacy and safety of 0.3% carbomer gel compared to placebo in patients with moderate-to-severe dry eye syndrome. Ophthalmology. 1997;104(9):1402-8. 5. Wozniak PA, Schmidl D, Bata AM, et al. Effect of different lubricant eye gels on tear film thickness as measured with ultrahigh-resolution optical coherence tomography. Acta Ophthalmol. 2017;95(4):e307-13. 6. Ubels JL, Edelhauser HF, Foley KM, et al. The efficacy of retinoic acid ointment for treatment of xerophthalmia and corneal epithelial wounds. Curr Eye Res. 1985;4(10):1049-57. 7. Terrie YC. Relieving dry eye: what every patient needs to know. Pharmacy Times. May 12, 2016. 8. Fogt JS, Fogt N, King-Smith PE, et al. Changes in tear lipid layer thickness and symptoms following the use of artificial tears with and without omega-3 fatty acids: a randomized, double-masked, crossover study. Clin Ophthalmol. 2019;13:2553-61. 9. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome study group. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900-7. 10. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. 11. Roberts CW, Carniglia PE, Brazzo BG. Comparison of topical cyclosporine, punctal occlusion, and a combination for the treatment of dry eye. Cornea. 2007;26(7):805-9. 12. Zhou L, Zhao SZ, Koh SK, et al. In-depth analysis of the human tear proteome. J Proteomics. 2012;75(13):3877-85. 13. Alio JL, Rodriguez AE, Ferreira-Oliveira R, et al. Treatment of dry eye disease with autologous platelet-rich plasma: a prospective, interventional, non-randomized study. Ophthalmol Ther. 2017;6(2):285-93. 14. Kim KM, Shin YT, Kim HK. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn J Ophthalmol. 2012;56(6):544-50. 15. Fea AM, Aragno V, Testa V, et al. The effect of autologous platelet lysate eye drops: an in vivo confocal microscopy study. Biomed Res Int. 2016;2016:8406832 16. Liang L, Li W, Ling S, et al. Amniotic membrane extraction solution for ocular chemical burns. Clin Exp Ophthalmol. 2009;37(9):855-63. 17. Murri MS, Moshirfar M, Birdsong OC, et al. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clinical Ophthalmol. 2018;12:1105-12. 18. Sheha H, Tighe S, Hashem O, Hayashida Y. Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol. 2019;13:1973-80. 19. Versura P, Giannaccare G, Pellegrini M, et al. Neurotrophic keratitis: current challenges and future prospects. Eye Brain. 2018;10:37-45. 20. Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye (Lond). 2003;17(8):989-995. 21. Bonini S, Lambiase A, Rama P, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. 2018;125(9):1332-43. 22. Oxervate (cenegermin-bkbj) ophthalmic solution 0.002% (20mcg/mL) [US package insert]. Boston, MA: Dompé US.; 2018. 23. Cheung AY, Shah AP, Pierson KL, et al. Use of cenegermin in the presence of bandage contact lenses. Cornea. 2022;41(1):78-82. |


