In eye care, there is a tendency to characterize ocular inflammation as something inherently negative that needs to be combated at all costs. In reality, however, the inflammatory response is part of the body’s natural self-defense and tissue repair system, and is required to ensure normal functioning of the eye.
When ocular tissue is exposed to an irritant or a perceived threat, the body responds by sending chemical mediators and inflammatory cells to clear the antigen, remove compromised tissue and assist in tissue reconstruction. There are many times indeed when we should be grateful rather than fearful.
These complex processes involve immune cells (e.g., lymphocytes, granulocytes, antigen-presenting cells) chemical mediators of ongoing inflammation (e.g., cytokines, prostaglandins, chemokines) and immune tissue (e.g., regional lymph nodes, secondary lymph tissue and primary lymph tissue such as the thymus).1 Potential catalysts are many, but the reaction most commonly follows injury, allergy, infection or autoimmunity.
The subsequent effect could be mild and self-contained or severe and destructive; the outcome depends on the trigger and host events. In cases when the inflammatory response is excessive—with potential for permanent damage to ocular tissue—or the patient is symptomatic, treatment becomes indicated. Fortunately, clinicians have myriad ophthalmic anti-inflammatories to consider, of which the two broad classes are of course the steroids and the NSAIDs.
This article will provide an overview of the available options and my clinical impressions of the decision-making process. Given the many inherent differences in practitioners’ comfort level and patients’ circumstances, individualization is a necessity and practice patterns will vary.
Corticosteroids
The most important class of topical anti-inflammatory medications, corticosteroids have been a staple of medical care since the early 1950s.2,3,6 Despite this long tenure, their exact anti-inflammatory mechanism is still not fully understood.3 What we do know is that nearly all cells of the body express a receptor for these chemicals (the glucocorticoid receptor, or GR), which helps explain the wide-ranging effects—and side effects—of this class of medications. 3,5-7
The primary anti-inflammatory mechanism of corticosteroids is likely their role in inhibiting cytokines and chemokines.2,3,5,7 Though these chemical mediators are a cellular byproduct of inflammation, they are also probably among the primary mediators of the entire inflammatory cascade. They promote activation, migration, proliferation and recruitment of immune cells.
Secondarily, steroids inhibit production of inflammatory molecules such as prostaglandins (PGs), promote stability of granulocytes such as mast cells and basophils, reduce permeability of vascular beds, and reduce both angiogenesis and fibrogenesis.2,5
While systemic steroids have a direct impact on the development and differentiation of immune-competent cells, topical steroids probably exert their effect by reducing local tissue permeability and decreasing production of cytokines and chemokines within local immune cells.2 Due to their broad range of target cells, corticosteroids have the widest (though least specific) anti-inflammatory effect of any topically used agent.
 | |
|
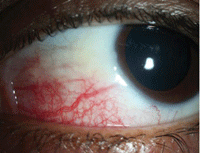
Sectoral episcleritis: Frontline treatment is an oral NSAID or topical steroid. With scleritis, topical steroids should generally be avoided
—in some subtypes, their use may accelerate scleral thinning by potentiating collagenase activity.
|
Mitigating to some extent the dramatically positive anti-inflammatory effect of glucocorticoids are their side effects. Though topically administered corticosteroids are better tolerated than systemic formulations, they have the well-known side effects within the eye of accelerating cataract formation, increasing intraocular pressure, increasing risk of infection or prolonging pre-existing infectious processes and may very rarely cause systemic effects such as increased blood sugar or psychological effects such as anxiety and insomnia. Therefore, use of topical ophthalmic steroids is a decision in which the balance of benefit and risk should be carefully assessed, as cavalier use may lead to substantial worsening of ocular health.
Complicating things further, penetration of glucocorticoids is impeded by the corneal epithelium. Lipophilic bases such as alcohols and acetates, and higher viscosity delivery vehicles that increase contact time generally increase intraocular penetration of most topical corticosteroids, and therefore increase aqueous concentrations.
However, in many cases of anterior segment use, it’s worth asking whether or not you want anterior chamber penetration of a steroid.3-6 After all, the weaker the steroid penetration, the less potential for IOP response and cataract development there is.
Lastly, each commercially available ophthalmic steroid has its own pharmacokinetic properties, strength of anti-inflammatory effect and propensity for steroid-induced side effects. Therefore, selection of an appropriate corticosteroid should derive from the full clinical picture, including:
• the site of inflammation (for example, intraocular penetration is unnecessary and ultimately unwanted in cases of inflammatory conjunctivitis or superficial keratitis)
• the magnitude of the inflammatory response
• the likelihood of inducing steroidal side effects within a patient, the possible magnitude of that side effect for a given agent and the patient-specific consequences of those effects
Below are broad overviews of several available preparations, in descending order of efficacy.
• Difluprednate emulsion (Durezol, Alcon). The newest ophthalmic corticosteroid on the market, difluprednate has the greatest theoretical anti-inflammatory effect and highest in vivo potency of available options.10 Anecdotally, I have used Durezol to good effect on eyes that have had inflammation recalcitrant to prednislone acetate 1%, including my own eye during flare-ups of uveitis. It also has the benefit of a more uniform distribution, with dosing based on the emulsion vehicle, and doesn’t require shaking prior to use, unlike the suspension-based steroids.
Does this increased potency affect the propensity for IOP response? Reports vary based upon treatment populations and study design. A large study showed only transient differences in IOP between prednisolone and difluprednate, while a smaller retrospective analysis of their use in iritis showed an IOP increase of 20mm Hg or more in 19% of patients recalcitrant to prednislone who were switched to difluprednate.14,15

|
|
|
Infiltrative keratitis responds well to treatment with corticosteroids. Loteprednol or FML are great choices in an eye like this.
|
The study design, treatment duration (as long as 16 weeks in some eyes) and population (7% of whom were on no steroid prior to beginning diflurprednate) limit broad application of these findings to other populations, but it highlights the importance of IOP monitoring when considering difluprednate for long periods.
Regardless, given its potency, there is no doubt Durezol is a useful part of the clinical armament, particularly in cases of moderate to severe intraocular inflammation or when only a short course of treatment is expected and with careful monitoring for IOP spikes when treatment duration is extended.
• Prednisolone acetate 1% (Pred Forte, Allergan or generic). This is a good general purpose, cortisone-based anti-inflammatory that most eye care providers are quite familiar with. Though increasing the concentration from 0.25% to 1% increases its efficacy, any further increase in concentration would yield no benefit; therefore, 1% is the highest concentration available commercially.4,5
It is well described in eye care that the branded version of the medication exhibits superior distribution and dose uniformity within the suspension than the generic, although both require re-suspension with shaking.16 Both branded and generic prednisolone acetate are good steroid choices for many forms of anterior segment inflammation, though severe cases may occasionally require a greater potency medication.
• Dexamethasone. The steroidal king of the combination drugs, dexamethasone is also one of the most potent anti-inflammatories of all corticosteroids. Though the molecule of dexamethasone has roughly six times the anti-inflammatory effect of prednisolone, its topical formulation is 10 times more dilute (prednisolone acetate 1% vs. dexamethasone 0.1%) and does not penetrate into the anterior chamber as readily.3-5 Thus, topical prednisolone acetate 1% is a slightly more potent corticosteroid as dosed.4,5 The tendency of IOP response with dexamethasone is thought to be similar to that of prednisolone acetate.
• Fluorometholone. Unlike prednisolone’s cortisol base, fluorometholone is progesterone derived. The 0.1% version of the drug is roughly equivalent in efficacy to and better in safety than the 0.25% version. As a result, there is limited rationale for choosing FML in its higher-concentration form.5
Fluorometholone is reported to be somewhat weaker than prednisolone acetate both in vitro and within the anterior chamber.3 It does not penetrate as readily into the chamber, although in certain ocular surface scenarios it is generally reported to be equivalent.4 Fluorometholone has a lower incidence of IOP spike than prednisolone acetate.5,9
Because of its enhanced safety profile over prednisolone acetate and roughly equivalent anti-inflammatory effect when used at the ocular surface, it is a good alternative to prednisolone acetate or (when cost is an issue) loteprednol for ocular surface disease. Currently, availability of fluorometholone has been limited in some (but not all) areas of the country, as a handful of its manufacturers have ceased producing it.
• Loteprednol etabonate (Lotemax, Alrex, Bausch + Lomb). This ester-based steroid was developed in the early 1990s as an alternative to prednisolone acetate with a better safety profile.5,11 Excess unbound loteprednol molecules undergo metabolic transformation after a relatively short time, resulting in fewer unwanted side effects such as increased IOP and cataract development.
Theoretical and clinical efficacy of the original loteprednol products has been shown to be quite good, lagging only slightly behind that of prednisolone acetate.5,10,11 Its safety profile has also been clinically shown to be good, with a lower degree of IOP spikes in the setting of known steroid responders.5,11,13
The recent change of Lotemax from a suspension to a gel delivery increases uniformity between drops and increases maximum tissue concentrations within the cornea and conjunctiva compared to the suspension form.11 Given its safety and anti-inflammatory profile, loteprednol is a terrific option in most forms of ocular surface and moderate forms of deeper inflammation. Out-of-pocket cost to the patient in some circumstances may need to be addressed.
Non Steroidal Anti-Inflammatories
Where can you turn when you want the benefit of an anti-inflammatory without the steroid-associated eye effects? NSAIDs, of course.
As mentioned above, one effect of corticosteroids is inhibition of pro-inflammatory molecules such as prostaglandins, a group of eicosanoids derived from arachidonic acid via the cyclo-oxygenase enzymes COX-1 and COX-2.5,12
COX-1 is an enzyme that plays a role in mediation of physiologic function. Therefore, the PGs that it produces are created under normal circumstances. COX-2, on the other hand, mediates production of pro-inflammatory PGs. The exact mechanism that PGs play in the inflammatory cascade is not fully understood, but it appears they primarily enhance vascular permeability and also sensitize pain receptors.5,12
As NSAIDs are inhibitors of COX enzymes, they reduce their inflammatory product —prostaglandins. Given that inhibition of PGs is only one of the many anti-inflammatory effects of steroids, it’s no surprise that they have a broader effect in suppressing inflammation than NSAIDs. That’s not to say that steroids are superior in all ways, however. A number of studies show NSAIDs are objectively more effective in helping reestablish the blood-aqueous barrier than glucocorticoids, and pairing the two drugs may yield even greater benefit.12

| |
|
A pyogenic granuloma. These capillary-based, reactionary growths often respond well to treatment with topical corticosteroids.
|
Excess PG production has been strongly implicated in a number of retinal disorders, such as diabetic macular edema and the development of choroidal neovascular membranes.12 Given the role PGs seem to play in enhancing vascular permeability, perhaps it’s no surprise that NSAIDs are widely used in the prevention and treatment of cystoid macular edema. For anterior segment use, NSAIDs are useful for their analgesic effect—which is not paired with an anesthetic effect, unlike proparacaine and similar drugs.17 This makes NSAIDs useful for managing pain with corneal trauma, as the analgesia provides some pain relief without compromising healing of the cornea.5,12
At my clinic, we use topical NSAIDs with our PRK patients in the postoperative period to help alleviate pain, as well as in other painful presentations. Of course, some eyes are more sensitive than others, so NSAIDs may not be sufficient for pain control in some cases of corneal abrasion.
While the different commercial preparations of corticosteroids have quite different properties given their varied anti-inflammatory effects, permeability and side effect profiles, NSAIDs as a class seem to be a bit more uniform. Permeability and half-life are both enhanced with nepafenac (a prodrug) and bromfenac, and only ketorolac is approved for allergic conjunctivitis, but all perform somewhat comparably at their recommended dosages. Of the group, only flurbiprofen 0.03% has been shown to be less effective than others in the treatment of cataract surgery-induced inflammation.12
As stated, the primary benefit of NSAIDs is to offer some of the anti-inflammatory effects of cortiocosteroids without their side effects. Interestingly, despite PG analogs being widely employed for glaucoma therapy, there is no apparent net effect on IOP with the ophthalmic use of NSAIDs, whose chief mechanism is to reduce PG, and they have not been linked to cataract formation. NSAIDs are generally quite safe, though reports of corneal melts have rarely been reported. Most typically these events have been attributed to generic diclofenac, but the event has been reported with all members of the ophthalmic NSAID class except flurbiprofen.12
Conclusion
Though not all ophthalmic inflammatory events require pharmaceutical intervention, you will be frequently required to prescribe an anti-inflammatory. Thankfully, the number and diversity of medications at our disposal should allow clinicians to prescribe case-specific anti-inflammatories, where extent and location of inflammation are taken into account. As a result, the patient is subsequently offered the safest available option.
1. Bouchard C. The Ocular Immune Response. In: Krachmer JH, Mannis MJ, Holland EJ eds. Cornea. 2nd ed. St Louis: Mosby;2004:59-93.
2. BenEzra D. Immunosuppression and immunomodulation. Ocular Inflammation: Basic and Clinical Concepts. BenEzra D, Ed. Martin Dunitz 1999:1-24.
3. McGhee CN. Pharmacokinetics of ophthalmic corticosteroids. British Journal of Ophthalmology. 1992;76:681-4.
4. McGhee CH, et al. Penetration of Synthetic Corticosteroids into Human Aqueous Humor. Eye. 1990;4:526-530.
5. Jaanus SD, et al. Antiinflammatory Drugs. In: Bartlett JD and Jaanus SD eds: Clinical Ocular Pharmacology. 4th Ed. Butterworth-Heinemann;2001:265-314.
6. Greaves MW. Anti-inflammatory action of corticosteroids. Post Graduate Medical Journal. 1976;52:631-3.
7. Van der Velden VH. Glucocorticoids: mechanism of action and anti-inflammatory potential in asthma. Mediators of Inflammation. 1998;7:229-37.
8. Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986-1000.
9. Morrison E, Archer DB. Effects of fluorometholone (FML) on the intraocular pressure of corticosteroid responders. British Journal of Ophthalmology. 1984;68:581-4.
10. Weiner G. Savvy Steroid Use. American Academy of Ophthalmology www.aao.org/publications/eyenet/201302/feature.cfm
11. Coffey MJ, DeCory HH, Lane SS. Development of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic drop. Clinical Ophthalmology. 2013;7:299-312.
12. Kim SJ, et al. Nonsteroidal Anti-inflammatory Drugs in Ophthalmology. Survey of Ophthalmology. 2010;55:108-133.
13. Bartlett JD, et al. Intraocular pressure Response to Loteprednol Etabonate in Known Steroid Responders. Journal of Ocular Pharmacology. 1993;9:157-165.
14. Birnbaum, AD et al. Elevation of Intraocular Pressure in Patients With Uveitis Treated With Topical Difluprednate. Arch Ophthalmol. 2011;129:664-676.
15. Foster CS, et al. Durezol (Difluprednate Ophthalmic Emulsion 0.05%) compared with Pred Forte 1% ophthalmic suspension in the treatment of endogenous anterior uveitis. J Ocul Pharmacol Ther. Oct 2010;26(5):475-483.
16. Stringer W, Bryant R. Dose. Uniformity of topical corticosteroid preparations: diflurprednate ophthalmic emulsion 0.05% versus branded and generic prednislone acetate ophthalmic suspension 1%. Clinical Ophthalmology. 2010; 4:1119-1124.
17. Mycek MJ, et al. Antiinflammatory Drugs. In Harvey RA and Champe PC eds, Pharmacology 2nd Ed. Lippincott Williams and Wilkins. 401-418.


