Creating a specialty contact lens fit for a cornea with a significant amount of irregular astigmatism, scarring or advanced ectasia can be a daunting task for many practitioners. Fitting a scleral lens on a congenitally enlarged cornea, a condition otherwise known as megalocornea, may prove to be even more intimidating for some. Megalocornea is a rare, predominantly X-linked developmental condition characterized by a non-progressive bilateral enlargement of the cornea that is typically larger than 12.5mm in horizontal visible iris diameter (HVID).1
As these patients are generally at higher risk for more complex ocular complications, a successful scleral lens fit can be life-changing. By understanding the basic principles of an optimal scleral lens fit, being observant behind the slit lamp and becoming familiar with your scleral lens fitting set, fitting this modality on a megalocornea can be much more straightforward than most clinicians think. The following case report will show you where to start and walk you through the process.
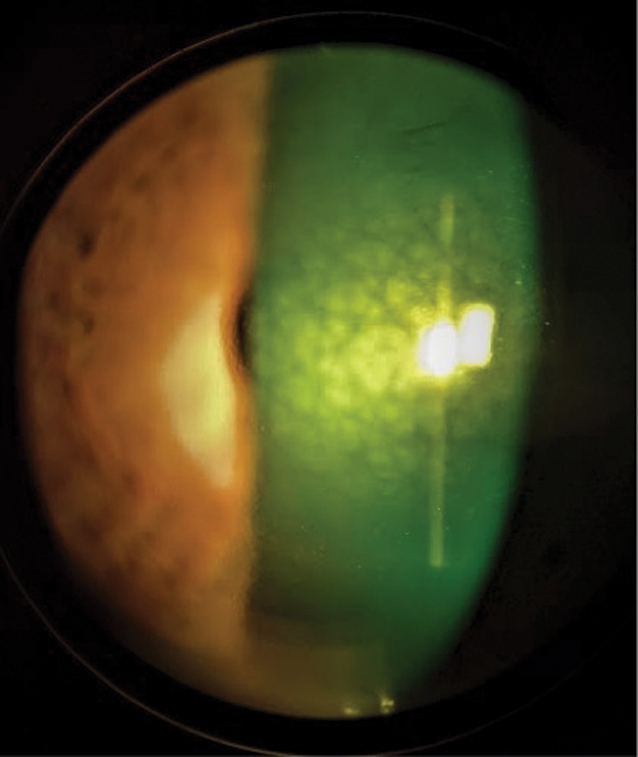 |
Fig. 1. This central mosaic dystrophy pattern is associated with megalocornea. Click image to enlarge. |
The Breakdown
There are several conditions that present similarly to megalocornea but differ upon closer inspection. One is primary congenital glaucoma with buphthalmos, a term used to describe the enlargement of the eyeball detected at birth or soon after due to uncontrolled glaucoma in early infancy.2
When megalocornea is present at birth, the HVID is typically 13mm or larger in the newborn.3 Unlike buphthalmos, megalocornea does not present with elevated intraocular pressure (IOP), corneal edema, opacification or Haab’s striae, which are horizontal, curvilinear breaks in Descemet’s membrane caused by elevated IOP.4 Megalocornea is typically symmetric in appearance, whereas congenital glaucoma can display asymmetry.5
Another condition that presents similarly to megalocornea is keratoglobus, a non-inflammatory, progressive generalized thinning (or steepening) and global ectasia of the cornea. Unlike keratoglobus, though, megalocornea does not present with generalized peripheral thinning or steepening of the corneal structure and is found earlier in life.6
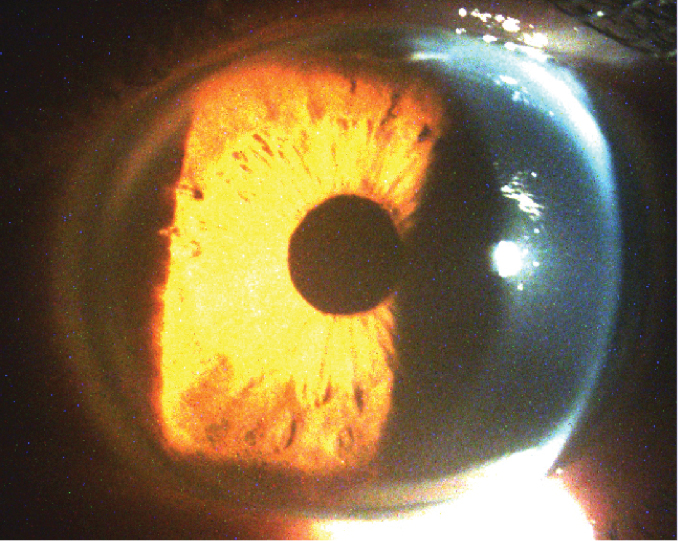 |
Fig. 2. A large HVID and deep anterior chamber are both characteristic signs of megalocornea. Click image to enlarge. |
As over 90% of megalocornea cases are X-linked recessive, most affected individuals are male.1,4 Myopia and with-the-rule astigmatism, but not amblyopia, have been associated with the condition in children. Those with the condition are also more susceptible to posterior vitreous and retinal detachments. When megalocornea is diagnosed, differentiation must be made between simple, or pure, megalocornea and megalophthalmus anterior.4,5,7
Simple megalocornea is known to have the following findings: bilateral HVIDs greater than 13mm, deep anterior chambers, normal IOP, normal central and peripheral corneal thicknesses, clear stromal tissue or central mosaic dystrophy, posterior positioning of the iris-lens diaphragm and a shortened vitreous cavity and body.7
Megalophthalmos anterior, on the other hand, combines all the signs of simple megalocornea with several additional abnormal findings, including iridodonesis, phacodonesis, ectopia lentis, early cataract and widened ciliary body band.7 As this subtype involves movement of the iris and crystalline lens, these patients have a higher risk of developing pigment dispersion glaucoma (as opposed to congenital glaucoma in buphthalmos) due to Krukenberg spindles, trabecular meshwork hyperpigmentation and iris transillumination defects.3,7
Megalocornea often occurs as an isolated condition but can also be a presenting sign of a larger developmental disease, such as Alport syndrome, a rare, inherited disorder that damages the tiny blood vessels of the kidneys.7-9 Marfan syndrome, the second most common inherited connective tissue disorder, is another condition that has been linked to megalocornea.10,11
Megalocornea is also associated with Down syndrome, or trisomy 21, one of the most common genetic diseases, which is marked by a characteristic facial appearance, short stature, intellectual disability and a host of developmental, ocular and cardiac abnormalities.9,12 Ehlers-Danlos syndrome, a connective tissue disorder that affects the skin, bones, blood vessels and many other organs and tissues, may also be associated with this corneal condition.13
Osteogenesis imperfecta, otherwise known as brittle bone disease, develops from a defect in the gene that produces collagen and is linked to megalocornea. These patients have fragile bones that fracture from very minor trauma and signs associated with a blue sclera.14,15 Finally, progressive facial hemiatrophy, renal carcinoma and mental handicap have also been documented in the literature as having a connection with megalocornea.9
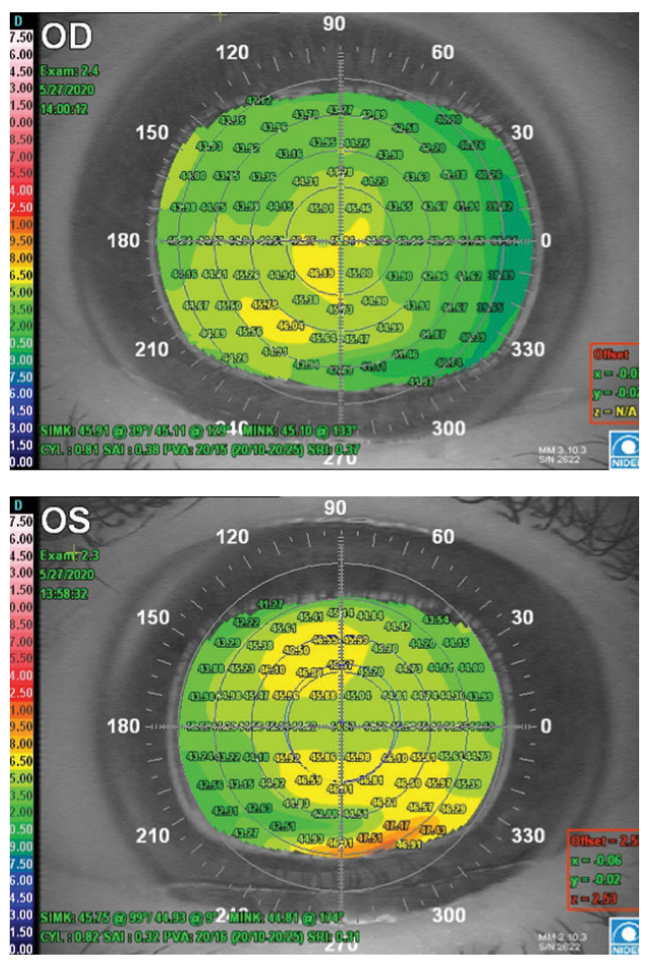 |
Fig. 3. These placido disc topographies of megalocornea have a generally uniform appearance OD (top) and mild peripheral inferior steepening OS (bottom). Click image to enlarge. |
The Case
A 57-year-old Caucasian male presented with constant blur in his left eye due to a recent spontaneous subluxation of an intraocular lens (IOL) without trauma. His medical history included migraines and chronic lower back pain, and his ocular history included megalocornea, metallic foreign body in his left eye and uneventful bilateral phacoemulsification during his late 40s.
Shortly after the lens subluxated, the patient saw a vitreoretinal surgeon who determined IOL removal would be the patient’s best option without an iris-fixed IOL replacement. He was subsequently referred to a contact lens specialist prior to intraocular surgery.
Wearing his habitual spectacle correction, the patient’s distance visual acuities were 20/20- OD and 20/400 that pinholed to 20/60- OS. There was no afferent pupillary defect noted, and IOPs were 10mm Hg OD and 11mm Hg OS. Manifest refraction yielded +1.00+0.75x055 with visual acuity of 20/20 OD and +7.25+0.75x120 with visual acuity of 20/20- OS.
Interestingly, during subjective refraction of the patient’s left eye, the subluxated lens partially descended into the visual axis and caused him to have to tilt his head to reposition the lens out of the visual axis. Although manifest refraction may have shown a partial contributory effect of the subluxated IOL, there was no reduction in the patient’s best-corrected visual acuity.
Slit lamp examination revealed bilateral enlarged corneas with moderately dense central mosaic dystrophy, diffuse pigmentation of the endothelium and a deep anterior chamber (Figures 1 and 2). Both corneas showed well-healed cataract incisional scars, and the left had a small subepithelial scar at 8 o’clock. The anterior chambers were quiet, and there were no signs of vitreous prolapse in the left eye. There was a well-centered posterior chamber IOL with a clear capsule in the right eye and a superonasally decentered posterior chamber IOL in the left.
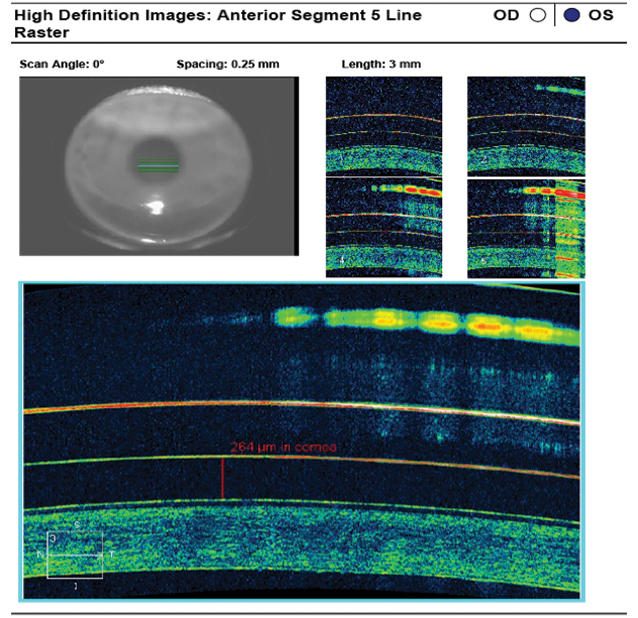 |
Fig. 4. AS-OCT of the 4300µm/38/44/8.4mm/16.0mm scleral lens reveals ideal apical tear film thickness after one hour of lens settling. Click image to enlarge. |
Dilated fundus exam revealed syneresis of the vitreous in the right eye and a posterior vitreous detachment in the left. The optic cups were small with pink neuroretinal rims, the maculae were flat and intact with even pigmentation and the peripheral fundus was unremarkable bilaterally.
The topographical map of the patient’s right eye showed an enlarged cornea with a uniform appearance without pathological steepening. The topographical map of his left eye also showed a generally uniform appearance with mild peripheral inferior steepening that was unable to be fully captured by topography (Figure 3). Simulated keratometer readings from the topographer were 45.11/45.91 at 039 and 44.93/45.75 at 099 of the right and left corneas, respectively. The HVIDs were 14.32mm OD and 14.56mm OS on topography.
Due to the high hyperopic prescription and the need for a contact lens with a larger diameter to vault the patient’s megalocornea, the SynergEyes VS scleral lens was selected for the initial diagnostic fitting.
Diagnostic Fitting
The initial diagnostic lens used for the patient’s left eye had parameters of 3600µm/36/42/8.4mm/16.0mm in the SynergEyes VS scleral design. This lens showed heavy edge lift of the scleral landing zone at both the flat and steep meridians. A cobalt blue penlight showed heavy central bearing. The patient immediately reported discomfort, so the lens was removed after a quick gross examination and slit lamp assessment.
The second diagnostic contact lens used had parameters of 4000µm/40/46/8.4mm/16.0mm in the same lens design. Once inserted, the lens immediately showed improved overall vault, although moderate central bearing persisted. The scleral landing zone showed improved scleral alignment as well, but there was still a decent amount of edge lift at both the flat and steep meridians. The patient noted dramatically improved comfort, but the foreign body sensation remained.
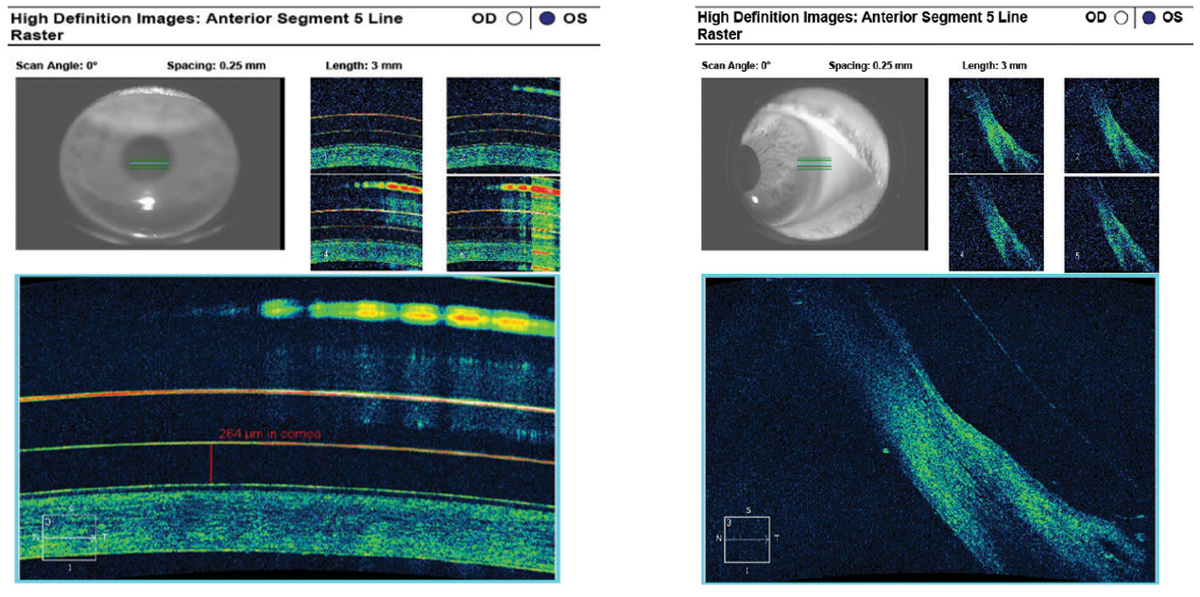 |
Fig. 5. AS-OCT reveals moderate edge lift at both the nasal (left) and temporal (right) sclera of the 4300µm/38/44/8.4mm/16.0mm scleral lens and mucosal debris in the tear layer. There is insufficient clearance at the limbus, indicating the scleral lens diameter and base curve are too small for this corneal HVID. Click image to enlarge. |
Although the tallest lens vault available in the SynergEyes VS diagnostic fitting set is 4000µm, the lens can be custom-ordered up to 4600µm.16 I determined that the tallest vault of the fitting set did not achieve clearance and discussed transitioning to a trial-and-error approach with the patient. As luck would have it, I remembered that our clinic had a custom 4300µm/38/44/8.4mm/16.0mm lens with -0.75D of sphere power from a previous order that had never been dispensed.
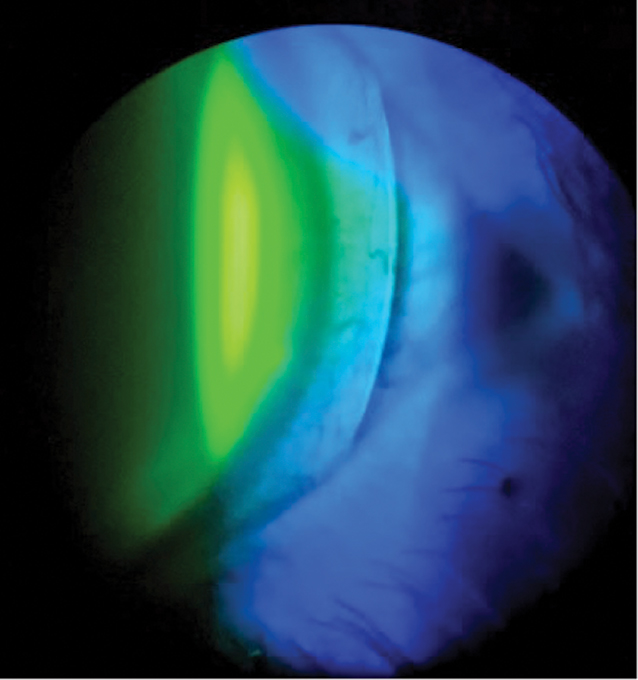 |
Fig. 6. The 4300µm/38/44/8.4mm/ 16.0mm scleral lens exhibits limbal bearing and mild edge lift of the scleral landing zone at the temporal sclera. Click image to enlarge. |
Once inserted, this third lens immediately showed apical clearance to alignment and from the nasal to temporal limbus. Although there was moderate edge lift at the scleral landing zone at each hour of the clock, this was to be expected. The patient reported immediate comfort, and we allowed the lens to settle on his eye for an hour prior to further evaluation. AS-OCT images were then captured to fine-tune the fit. Apical tear film thickness showed an ideal value of 264µm (Figure 4). As expected, the scleral landing zone showed edge lift at both the nasal and temporal sclera (Figure 5). More importantly, limbal bearing was present at both quadrants, indicating this scleral lens was not large enough for the patient’s corneal HVID and the base curve needed to be increased on the dispensing lens order.
With an overrefraction of +11.75+0.25x120 while wearing the most recent lens, the patient read 20/20-2 on the Snellen acuity chart. After vertexing the overrefraction, increasing the base curve to 8.8mm and incorporating the diagnostic lens power, the final lens power came out to +15.50D of sphere. During slit lamp evaluation, the lens showed apical alignment with mid-peripheral clearance. There was moderate bearing at the limbus at all hours of the clock and moderate edge lift at both the flat and steep scleral landing zones (Figure 6). The patient reported foreign body sensation but not pain or major discomfort. We ordered a new lens with parameters of 4300µm/42/48/8.8mm/17.5mm and +15.50D of sphere power with Menicon Z material to promote high oxygen permeability due to the extremely high plus power of the lens.
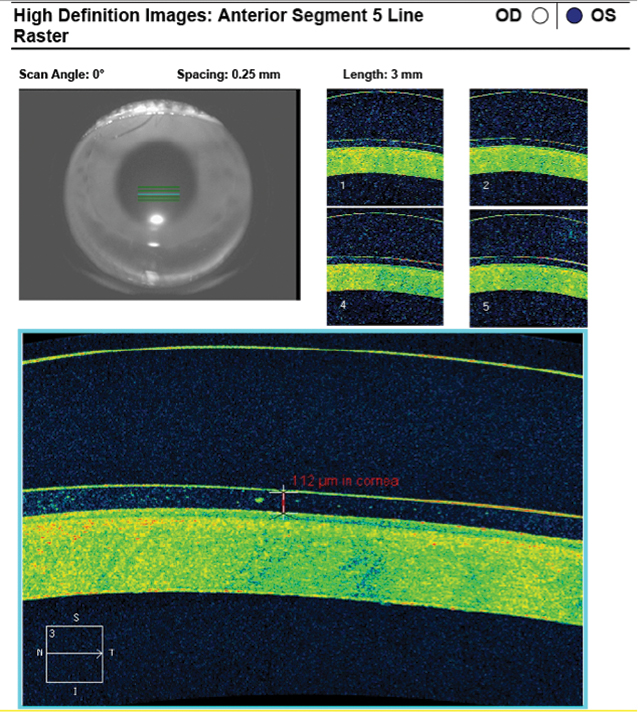 |
Fig. 7. AS-OCT of the 4300µm/42/48/8.8mm/17.5mm scleral lens shows a very high center thickness and apical tear film thickness of 114µm that is thin but acceptable after 60 minutes of lens settling. Click image to enlarge. |
Lens Dispensing
The patient returned for his first follow-up two months after the initial diagnostic fitting. He had seen a retina surgeon a month prior and undergone uneventful IOL removal. The patient was given clearance to wear sclerals about a month after the procedure.
The patient immediately noted a significant improvement in his vision with the new lens. On gross examination, the lens appeared to vault most of the megalocornea but showed signs of light bearing from 3 o’clock to 7 o’clock at the inferior limbus. AS-OCT images were captured after allowing the lens to settle for 60 minutes. Apical vault showed a thin but sufficient tear film thickness of 112µm after lens settling (Figure 7). Despite increasing the scleral landing zone by four points at both the flat and steep meridians of the diagnostic lens, it continued to show moderate edge lift at both quadrants over the sclera (Figure 8). Surprisingly, the patient reported the lens was very comfortable and did not note any foreign body sensation.
During visual acuity testing, the patient read a slow 20/40 line on the Snellen chart and accepted +1.25D of sphere power from an overrefraction that improved his visual acuity to 20/20-1. Although the patient was very satisfied with both the comfort and vision offered by the most current lens, I knew we could do even better with one more revision and placed an order with the following parameters: 4400µm/44/50/9.0mm/17.5mm with +17.75D of sphere power in the Menicon Z material.
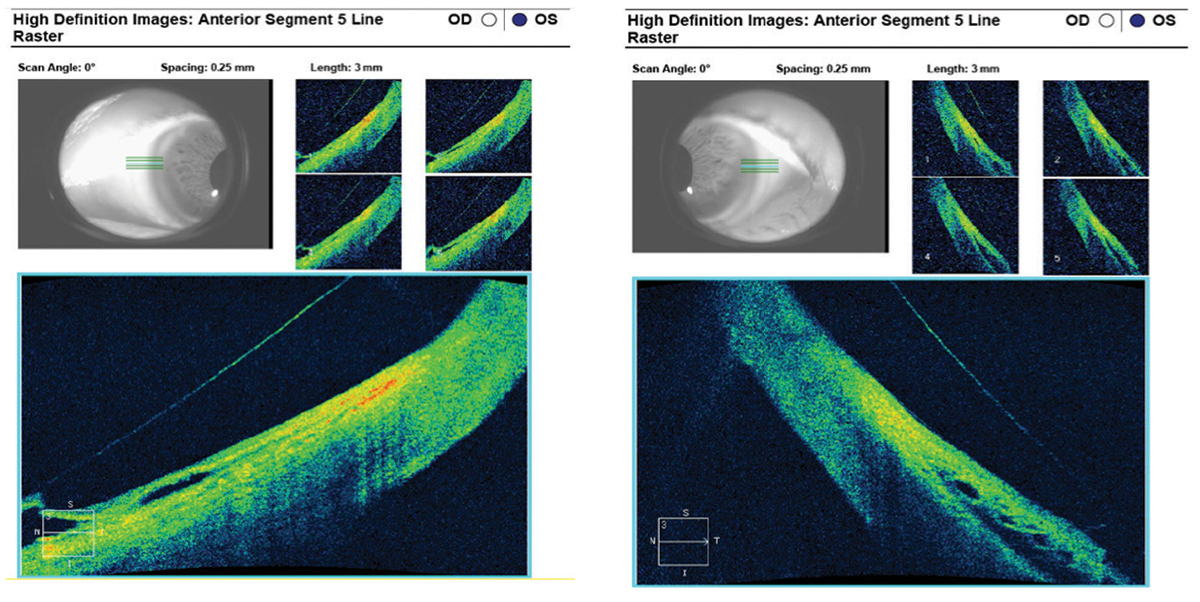 |
Fig. 8. The nasal (left) and temporal (right) scleral landing zone indicate moderate edge lift of the 4300µm/42/48/8.8mm/17.5mm scleral lens on AS-OCT. Click image to enlarge. |
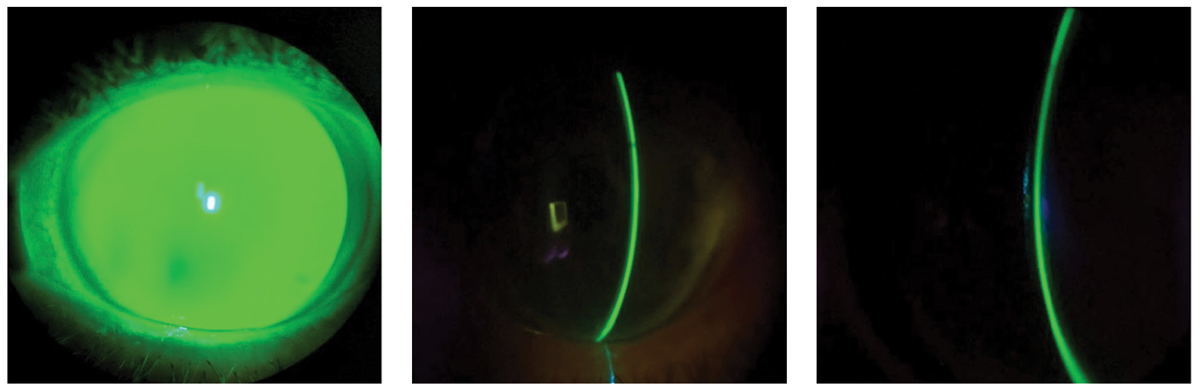 |
Fig. 9. The diffuse beam (left) and optic sections (center and right) on slit lamp examination of a scleral lens filled with preservative-free 0.9% NaCl solution and fluorescein dye show apical alignment with peripheral clearance from limbus to limbus. Click image to enlarge. |
Follow-Up
After a little over a month, the patient returned for his second follow-up. He reported he had been able to wear the lens comfortably for over 12 hours a day and had no issues with lens removal in the evening. After inserting the newest lens, the patient immediately reported great comfort and vision. There was adequate lens vault on broad beam and optic section from limbus to limbus (Figure 9). AS-OCT images were taken after 60 minutes of lens settling.
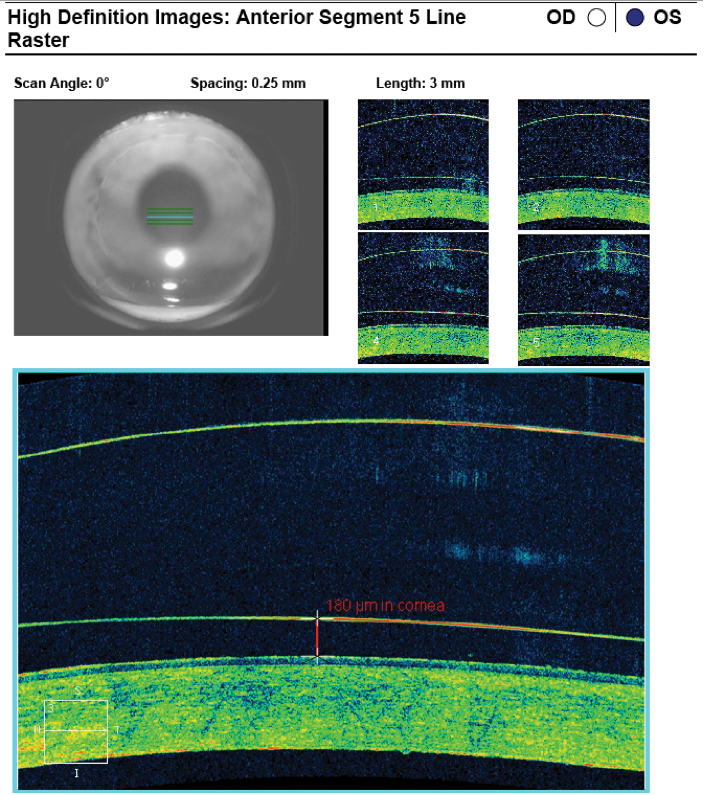 |
Fig. 10. AS-OCT revealed an ideal tear film thickness of 180µm at the apex after lens settling with the 4400µm/44/50/9.0mm/17.5mm scleral lens. Click image to enlarge. |
AS-OCT showed an ideal tear film thickness of 180µm at the apex after lens settling (Figure 10). Both the nasal and temporal scleral landing zones showed mild edge lift on slit lamp examination and AS-OCT (Figures 11 and 12). On visual acuity testing, the patient read 20/20-2 on the Snellen chart and did not accept an overrefraction. NaFl evaluation on the slit lamp showed apical alignment, midperipheral clearance and minimal touch at the limbus.
As the patient expressed great satisfaction, comfort and vision, we dispensed the lens to him. We reminded him to continue with his current contact lens hygiene regimen of Clear Care (Alcon) peroxide cleaner for lens disinfection and 0.9% preservative-free NaCl solution for lens insertion with a vented DMV inserter and to immediately discontinue scleral lens wear should he experience any ocular hyperemia with associated pain, discomfort or hazy or decreased vision.
Discussion
When initially diagnosed, megalocornea is a condition that necessitates further testing from both an ocular and systemic standpoint. From an ocular standpoint, the diagnosis must be further differentiated between simple megalocornea and megalophthalmos anterior. If the latter is suspected, further testing must be performed routinely to monitor for pigment dispersion glaucoma, ectopia lentis and early cataract.
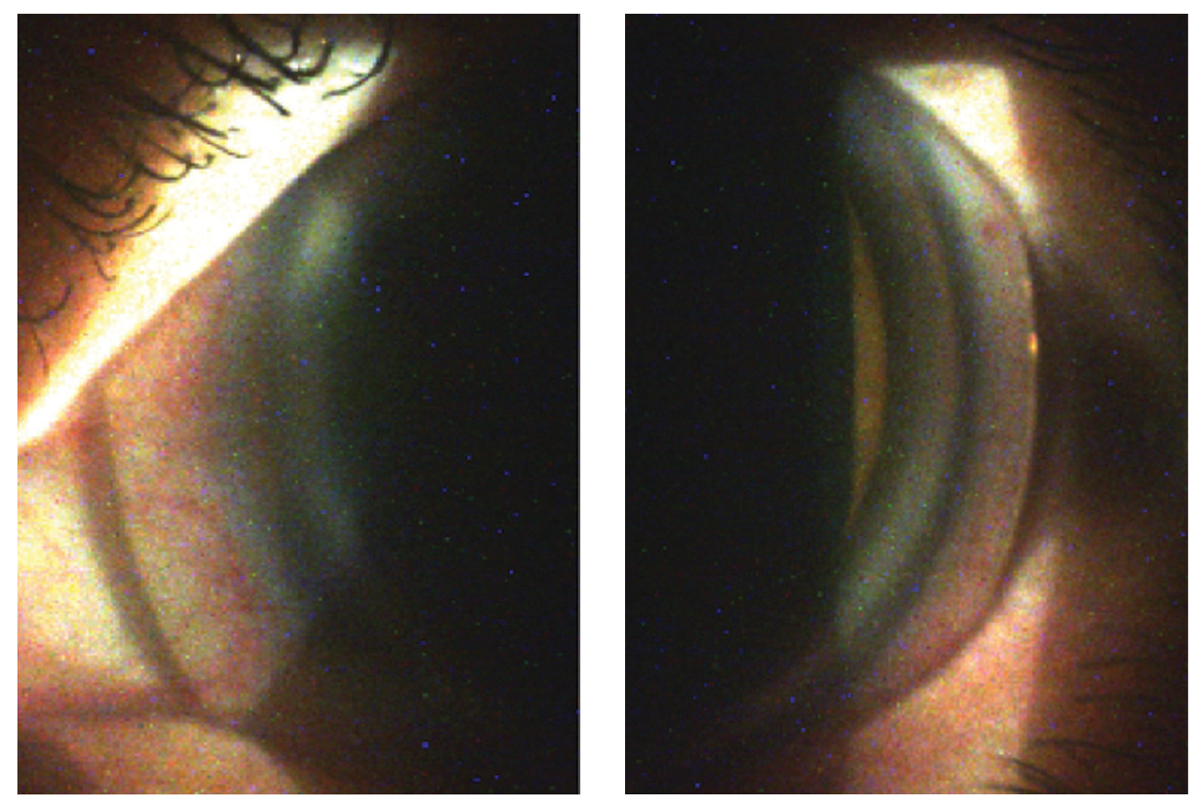 |
Fig. 11. Despite the nasal (left) and temporal (right) edge lift findings of the 4400µm/44/50/9.0mm/17.5mm scleral lens on slit lamp examination, the patient reported excellent comfort with wear. Click image to enlarge. |
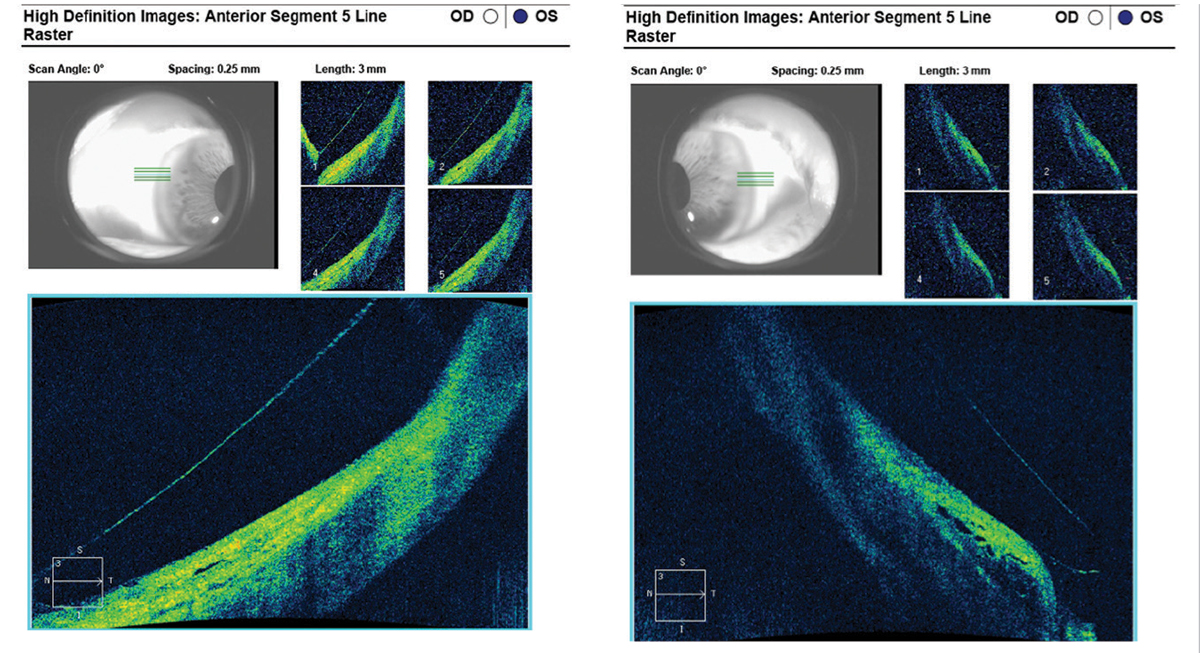 |
Fig. 12. With modified SynergEyes VS scleral lens parameters, the scleral landing zone showed ideal alignment at the nasal quadrant (left) and mild edge lift on the temporal sclera (right) on AS-OCT. Click image to enlarge. |
In addition, extra precaution must be taken if phacoemulsification is to be performed on these corneas due to the enlarged capsular bag and weakened zonules. Anterior or posterior iris-fixed IOLs may be necessary, as the enlarged anterior chamber, enlarged capsular bag and weakened zonules all pose a challenge to standard IOL placement.
From a systemic standpoint, the optometrist may have to consider referral to a nephrologist, rheumatologist, orthopedist or cardiologist if any underlying associated systemic conditions are suspected. A megalocornea diagnosis in a young patient should automatically trigger a developmental evaluation by a pediatrician. Overall, the prognosis of simple megalocornea is good.
As for the case study, the correct diagnosis for this patient is megalophthalmos anterior without pigment dispersion glaucoma and systemic involvement.
This scleral contact lens fitting was unique in several ways. First, the patient’s megalocornea required an extremely tall lens vault despite having average-to-steep keratometry readings on the topographer. Second, as the patient is aphakic, he required a scleral lens that is available in a very high plus power and a hyper-dK material to promote oxygen permeability. In addition, the patient’s scleral profile required a very steep scleral landing zone at both the scleral flat and steep meridians. In fact, the patient’s scleral profile was so steep that there was residual edge lift at the steep meridian despite having maxed out the scleral landing zone parameter in the manufacturer’s guidelines.16
The SynergEyes VS scleral lens was selected for this fitting because it offered desirable qualities and customizable parameters for this patient’s unique corneal condition and aphakic status.
Dr. Chung practices at the North Florida/South Georgia Veterans Health Administration, where he teaches externs and residents and serves as the lead contact lens fitting specialist.
1. Barnes J, Hopping G, Vaidyanathan U, et al. Megalocornea. American Academy of Ophthalmology. eyewiki.aao.org/Megalocornea. Updated June 14, 2019. Accessed January 13, 2021. 2. Feroze KB, Patel BC. Buphthalmos. StatPearls. www.ncbi.nlm.nih.gov/books/NBK430887/. Updated July 17, 2020. Accessed January 13, 2021. 3. Welder J, Oetting TA. Megalocornea. EyeRounds.org. webeye.ophth.uiowa.edu/eyeforum/cases/121-megalocornea.htm. Published September 17, 2010. Accessed January 13, 2021. 4. Marques ASA, Almeida AC. Megalocornea. American Academy of Ophthalmology. www.aao.org/image/megalocornea-7. Accessed January 13, 2021. 5. Harley R. Abnormalities of corneal size and shape: megalocornea and anterior megalophthalmos. In: Pediatric Ophthalmology. Philadelphia: W.B. Saunders; 1983, 468-71. 6. Keratoglobus: symptoms, causes, diagnosis, management and complications. American International Medical University. www.aimu.us/2018/02/25/keratoglobus-symptoms-causes-diagnosis-management-and-complications/. Published February 25, 2018. Accessed January 13, 2021. 7. Megalocornea. Eyedocs. www.eyedocs.co.uk/ophthalmology-articles/cornea/260-megalocornea-review-article. Accessed January 13, 2021. 8. Alport syndrome. MedlinePlus. medlineplus.gov/ency/article/000504.htm. Accessed January 13, 2021. 9. Kanski J, Bowling B. Kanski’s Clinical Ophthalmology, 5th ed. Philadelphia: Elsevier; 2017, 140. 10. Kay DB, Offutt ME. Marfan syndrome. American Academy of Ophthalmology. eyewiki.aao.org/Marfan_Syndrome. Updated January 6, 2020. Accessed January 13, 2021. 11. Marfan syndrome. MedlinePlus. ghr.nlm.nih.gov/condition/marfan-syndrome. Accessed January 13, 2021. 12. Rogers GL, Polomeno RC. Autosomal-dominant inheritance of megalocornea associated with Down’s syndrome. Am J Ophthalmol. 1974;78(3):526-9. 13. Ehlers-Danlos syndrome. MedlinePlus. ghr.nlm.nih.gov/condition/ehlers-danlos-syndrome. Accessed January 13, 2021. 14. Bober MB. Osteogenesis imperfecta (brittle bone disease). KidsHealth. kidshealth.org/en/parents/osteogenesis-imperfecta.html. Accessed January 13, 2021. 15. Osteogenesis imperfecta. Wikipedia. en.wikipedia.org/wiki/Osteogenesis_imperfecta. Updated January 8, 2021. Accessed January 13, 2021. 16. SynergEyes VS scleral fitting guide. SynergEyes. synergeyes.com/wp-content/uploads/2017/08/75270-Rev-A_SynergEyes-VS-Fitting-Guide.pdf. Accessed January 13, 2021. |


