The steady upswing in prescribing patterns and the resultant growth in market share of this evolutionary technology over the past decade has made it evident that eye care practitioners are recognizing the clinical advantages of silicone hydrogel (SiH) contact lenses.1 Yet, delivering optimum comfort and wearing time remains an ongoing challenge. The most common complaint is dryness—particularly among patients who are unable to wear their lenses for a full day and those who are at high risk for dropping out of lens wear.2
While it is the presence of silicone in silicone hydrogels which facilitates the extraordinary level of gas exchange, at the same time it also confers a hydrophobic nature to the material.3 To overcome this, manufacturers employ a variety of techniques to transform the contact lens into one that is compatible with the moisture-requiring environment of the ocular surface. These include surface treatments, embedded lubricating agents and utilizing longer polymer chains which have a natural affinity for water.
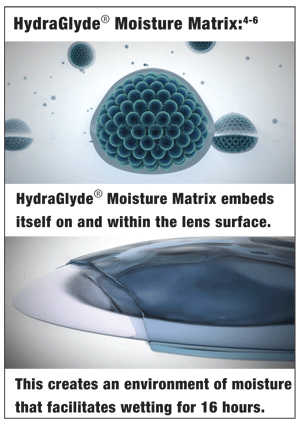 At the clinical level, we have also come to learn that the contact lens care solution can be an influential modifier of our patients’ lens wearing experience. To this end, Alcon Laboratories has developed and incorporated a unique wetting agent into its new OPTI-FREE® PureMoist® multi-purpose disinfectiong solution. A block copolymer, HydraGlyde® Moisture Matrix (poly[oxyethy lene]-poly[oxybutylene]), improves the wettability of SiH lenses by impacting both the lens surface wetting and bulk hydrating properties. With its excellent affinity for both the internal and external siloxane groups, HydraGlyde® Moisture Matrix functions to minimize the hydrophobic nature of SiH contact lenses.4
At the clinical level, we have also come to learn that the contact lens care solution can be an influential modifier of our patients’ lens wearing experience. To this end, Alcon Laboratories has developed and incorporated a unique wetting agent into its new OPTI-FREE® PureMoist® multi-purpose disinfectiong solution. A block copolymer, HydraGlyde® Moisture Matrix (poly[oxyethy lene]-poly[oxybutylene]), improves the wettability of SiH lenses by impacting both the lens surface wetting and bulk hydrating properties. With its excellent affinity for both the internal and external siloxane groups, HydraGlyde® Moisture Matrix functions to minimize the hydrophobic nature of SiH contact lenses.4
The ability of HydraGlyde® Moisture Matrix to embed itself on and within the lens surface was demonstrated in vitro through a captive bubble and lipophilic dye diffusion analysis. Captive bubble advancing contact angles were measured and compared for several major brands of SiH lenses, presoaked for 24 hours in Unisol® 4 and OPTI-FREE® PureMoist® MPDS.4
For most lenses tested, there was a statistically significant reduction in the advancing contact angles when the lenses were soaked with the OPTI-FREE® PureMoist® MPDS compared to the controls. Furthermore, all angles were less than 30°, which indicates that all the lens surfaces were rendered more hydrophilic following exposure to the OPTI-FREE® PureMoist® MPDS.
Lenses soaked in a lipophilic dye (Sudan IV), after having been pre-soaked in Unisol® 4 or the OPTI-FREE® PureMoist® MPDS, were analyzed for relative absorption of dye per lens amount. In all test cases, there was a reduction relative to the control in absolute absorbance of Sudan IV, which indicates that HydraGlyde® Moisture Matrix is able to effectively transform the lens bulk hydrophobic properties. Most importantly, there was a significant differentiation between the saline control and the OPTI-FREE® PureMoist® MPDS, even at the 16-hour time point. The 16-hour time point was used to simulate an end of the day lens wear exposure.4
Fortunately, with the newly developed OPTI-FREE® PureMoist® MPDS containing HydraGlyde® Moisture Matrix, we can now prescribe a care solution that incorporates an innovative surface-active agent specifically designed and optimized for silicone hydrogel lenses.
1. Morgan PB, Efron N, Helland M, et al. Twenty first century trends in silicone hydrogel contact lens fitting: an international perspective. Cont Lens Anterior Eye. 2010 Aug;33(4):196-8.
2. Schlanger JL. A study of contact lens failures. J Am Optom Assoc. 1993 Mar;64(3):220–224.
3. Dumbleton K. The Physical and clinical characteristics of silicone hydrogel lenses: how they work? Silicone Hydrogels. 2002 Oct. Available at: www.siliconehydrogels.org/editorials/previous_editorials_kathryn.asp (Accessed July 2011).
4. Davis J, Ketelson HA, Shows A, Meadows DL. A Lens Care Solution Designed for Wetting Silicone Hydrogel Materials. Poster presented at The Association for Research in Vision and Ophthalmology meeting, May 2-6, 2010; Ft. Lauderdale, Fla. (Alcon Research Ltd.)
5. Napier L, Garofalo R, Lemp J, Ketelson HA, Lally J. Clinical evaluation of an investigational multi-purpose disinfecting solution. Poster presented at CLAO; September 2010; Las Vegas, NV. (Alcon Research Ltd.)
6. Data on file, Alcon Research Ltd.


