This is an exciting time to be treating our patients who suffer from corneal ectasias. For patients with keratoconus, relatively new treatment options include scleral lenses and collagen cross-linking (CXL). For patients with failed corneal grafts, keratoprosthetics might offer a second chance at restoration of vision they wouldn’t otherwise have thought possible. This article will provide an overview of the technology available today for these three options.
Scleral Lenses
First introduced in the late 1800s, today’s modern scleral lens is easily reproducible and manufactured by several different companies.1,2
Scleral lenses have many indications, including primary and secondary corneal ectasias, post-corneal transplants, corneal scars, and corneal dystrophies or degenerations.3 Scleral lenses also can be prescribed for patients with severe dry eye, graft vs. host disease, Sjögren’s syndrome, Stevens-Johnson syndrome, neurotrophic keratopathy or chronic inflammatory conditions such as limbal stem cell deficiency and ocular cicatricial pemphigoid.3
Scleral lenses benefit patients with keratoconus by normalizing corneal irregularities. The fluid between the cornea and scleral lens creates a smoother surface, thus neutralizing an irregular corneal surface.4
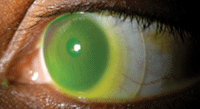
1. Scleral lens on a patient with keratoconus.
Depending on the amount of corneal ectasia, different scleral lens diameters may be used. Large diameter lenses are able to create a greater tear reservoir and provide more clearance between the lens and the cornea. This is useful if there is a significant difference in corneal sagittal height (ectasia). Large diameter scleral lenses have a wider area of bearing in the landing zone, which may improve lens comfort. Smaller diameter scleral lenses are easier to handle and may be used when there is less corneal ectasia. When fitting scleral lenses, the most important goal is to clear the cornea completely. In some cases, this can be accomplished through the smaller diameter lens. But keep in mind, scleral lenses should be incredibly comfortable so be flexible and switch between larger and smaller diameters to find the right fit.
When a larger diameter lens still does not provide complete corneal clearance, it may be helpful to make a larger change in sagittal depth. The amount of clearance needed varies with the condition. You may need a larger sagittal height in cases of keratoglobus, when rehabilitating patients with ocular surface disease or, as mentioned earlier, when a patient presents with a significant difference in ecstasia. On the other hand, post-corneal grafts or corneal scars may require a smaller sagittal height.
Corneal thickness may also be useful as a comparison and reference. For normal eyes, the average corneal thickness is 535µm centrally and 650µm peripherally. However, with corneal ectasias, central corneal thickness may be significantly thinner.
Corneal Collagen Cross-linking
Cross-linking frequently is used within the polymer industry to harden materials, and in bioengineering to stabilize tissue. In recent years, researchers have developed clinically effective protocols that use riboflavin and UV light to strengthen the cornea by increasing the crosslinks within the collagen fibers. CXL has been used internationally for more than a decade, but the FDA still considers it an off-label procedure. In the United States, a 10-center prospective, randomized clinical trial ran from December 2007 to April 2011 to determine the safety and effectiveness of corneal cross-linking performed in eyes with progressive keratoconus. Data has been collected and the results are pending.5
Although CXL halts the progression of corneal ectasia, causes flattening of keratometry measurements, and improves uncorrected and best-corrected vision, it does not fully correct refractive error or eliminate the need for glasses/contact lenses.
Investigations into the possibility of inducing cross-linking in the corneal stroma as a conservative treatment for keratoconus began in the mid-1990s. The biomechanical behavior of the cornea could be altered by irradiation using ultraviolet light with photosensitizers and through aldehyde reactions, as demonstrated by Eberhard Spoerl, Ph.D., and Theo Seiler, M.D., Ph.D.6
The porcine corneas were treated with glutaraldehyde, Karnovsky’s solution (glutaraldehayde and paraformaldehyde), or riboflavin and UV irradiation. These treatments caused an increase in corneal stiffness compared to untreated corneas.6 Riboflavin is a non-toxic photosensitizer comprised of vitamin B2, which is water-soluble. It can penetrate easily into the corneal stroma in the absence of the corneal epithelium.
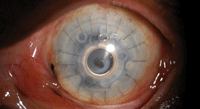
2. Boston keratoprosthesis.
CXL was first performed in 2003 by Gregor Wollensak, M.D., and colleagues.7 In patients with preoperative progressive keratoconus, CXL appeared to halt the progression of corneal ectasia.7 It has been documented that CXL causes flattening of keratometry measurements—from 1.45D to 2.68D at follow-up, depending on the study. There also tends to be improvements of uncorrected and best-corrected visual acuity with the flatting of the cornea (results vary).7,8 Untreated eyes had further steepening of keratometry readings and worsening of best-corrected visual acuity.9
Generally speaking, CXL is a safe and effective procedure. About 85% to 90% of UVA radiation is absorbed in the anterior 400μm of the cornea. CXL is not recommended for patients with corneas thinner than 400μm.10
• Epi-off CXL. The typical procedure for CXL involves applying a topical anesthetic, then removing 7mm of the central corneal epithelium to allow a uniform diffusion of riboflavin into the stroma.10 Next, riboflavin 0.1 % solution is applied prior to UVA irradiation to act as both a photosensitizer and a UV blocker.10 Homogenous UV irradiance of 3mW/cm2 and a wavelength of 370nm is used to irradiate the cornea for a 30-minute period. An antibiotic ointment is applied post-treatment until the cornea has reepithelialized.6
• Epi-on CXL. Cross-linking also can be performed without epithelial debridement. Studies are currently investigating whether the epithelium should be partially or completely removed during the cross-linking procedure. An advantage to leaving the epithelium intact is that both the procedure and the postoperative healing period is more comfortable for the patient.
The risk of infection may also be reduced with an intact epithelium. However, when the epithelium is intact, there may be an increase in procedure time, as it may take longer for the corneal stroma to absorb enough riboflavin. In fact, epithelial debridement may be needed to achieve stromal saturation of riboflavin during the procedure.6
Different techniques currently are being explored to make the epithelium more permeable. These can range from scratching to chemical treatment with topical anesthetics or preservatives to help the large riboflavin molecule more easily pass through the epithelium.
• Postoperative Care. After surgery, CXL healing generally has been shown to be unremarkable, with the exception of slight transient stromal edema until corneal re-epitheliazation.6 There are no changes associated with corneal or lens transparency, nor is there any evidence of cataract formation. Retinal damage is not observed with CXL. Additionally, CXL does not alter the ability to wear contact lenses postoperatively.
Topical antibiotics and anti-inflammatory drops are used after CXL. If the corneal epithelium is removed, a bandage contact lens may be indicated.
Stromal haze, however, has been reported after CXL treatment.11,12 In one study, the stromal haze developed between the second and third postoperative months and was resistant to topical steroids. Six months after CXL, stromal haze was unchanged and did not impair best-corrected visual acuity postoperatively. Conversely, stromal haze did impair best-corrected and uncorrected visual acuity. Thinner corneas and reticular hypo-reflective microstriae demonstrated by confocal analysis were risk factors for stromal haze. The haze may be associated with the depth of the cross-linking procedure and the amount of keratocytes lost.6,11 Patients with advanced keratoconus are at higher risk of haze development due to their thinner corneas and steeper corneal curvatures.11
Another finding after CXL is a thin stromal demarcation line over the entire cornea at a depth of approximately 300μm.13 The demarcation line is visible beginning two weeks after treatment and does not cause any changes in the corneal endothelium, the lens or intraocular pressure. The stromal demarcation line may be due to changes in the refractive index between the untreated and treated cornea, or may be due to the reflection properties of treated and untreated corneas.11
CXL may be able to delay or help avoid corneal grafts in patients with keratoconus. It may also be able to create a cornea more receptive to contact lenses and improve the functional refraction with contact lenses.
After CXL, you must address the patient’s refractive error. A refraction for glasses and a contact lens fitting should be performed. Keep in mind that the curvature of the cornea may change over months, so repeated refractions, corneal topographies and contact lens adjustments must be performed.
Keratoprosthetics
A synthetic (or partially synthetic) keratoprosthetic device can replace an opaque human cornea to provide a clear view through the front of the eye. In the keratoprosthesis procedure, a severely damaged or diseased cornea is surgically replaced with an artificial cornea. This procedure is used for severe corneal opacities, failed corneal transplants, or when standard corneal transplants are unlikely to succeed.14
Keratoprosthetics are made of clear plastic with excellent tissue tolerance and optical properties. They vary in design, size and implantation techniques, but consist of three parts and, when fully assembled, have the shape of a collar button.
The two devices currently approved for use in the United States are AlphaCor (Addition Technology) and the Boston keratoprosthesis (Boston KPro).
• AlphaCor. Made of pHEMA, AlphaCor consists of two parts: a transparent, low-water content central core and a cloudy, high-water content outer porous skirt.
The AlphaCor procedure is executed in two stages, performed approximately three months apart. In the first part, a 180º degree incision is used to place the implant within the central portion of the diseased cornea. The outer conjunctiva is then placed over the implant to assist healing. Three months later, the outer half of the cornea is removed to provide a clear view into the eye.15
• Boston keratoprosthesis. Developed by Claes Dohlman, M.D., Ph.D., the Boston keratoprosthesis (also referred to as the KPro) consists of a central PMMA plastic button with a surrounding human donor cornea skirt. In the one-time procedure, the device is inserted into a corneal graft and then sutured into the patient’s cornea. If the crystalline lens is present, it is removed. A soft contact lens is then used to bandage the surface. The donor cornea is placed on the front collar button and a titanium screw locks the KPro device into proper alignment.
If the eye is otherwise healthy, vision should return more rapidly than with the AlphaCor procedure. KPro currently is the most frequently used artificial cornea both nationally and internationally.
Potential complications with artificial cornea procedures include infection, device melting, hemorrhage during surgery, worsening glaucoma, acute retinal necrosis, chronic hypotony and poor visual potential if the retina and optic nerve are unhealthy.16-18
Next time you encounter a case of clinically significant corneal ectasia, take comfort in knowing that there are several promising options to help that patient. Scleral lenses have been a tremendous benefit, with the added advantage of offering a non-invasive and reversible solution. Although collagen cross-linking is not yet FDA approved, you may want to preemptively create a list of patients who may benefit from the procedure upon its eventual FDA approval. Finally, patients with failed corneal grafts are no longer bereft of options—a keratoprosthesis may allow for meaningful improvement in vision.
Dr. Barnett is a principal optometrist at the UC Davis Medical Center in Sacramento, where she performs primary care and eye examinations and fits contact lenses including specialty lenses. She also lectures on optics and contact lenses to ophthalmology residents.
1. Ezekiel DF. Gas permeable haptic lenses. J Br Contact Lens Assoc. 1983 Oct;6(4):158-61.
2. Rosenthal, P, Croteau A. Fluid-ventilated, gas-permeable scleral contact lens is an effective option for managing severe ocular surface disease and many corneal disorders that would otherwise require penetrating keratoplasty. Eye Contact Lens. 2005 May; 31(3):130-4.
3. van der Worp E. A guide to scleral lens fitting [monograph online]. Scleral Lens Education Society. 2010. Available at
http://commons.pacificu.edu/mono/4. Accessed July 2012.
4. Schornack MM, Patel SV. Scleral lenses in the management of keratoconus. Eye Contact Lens. Jan 2010;36(1):39-44.
5.
6. Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998 Jan;66(1):97-103.
7. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003 May;135(5):620-7.
8. Vinciguerra P, Albe E, Trazza S, et al. Intraoperative and postoperative effects of corneal collagen cross-linking on progressive keratoconus. Arch Ophthalmol. 2009 Oct;127(10):1258-65.
9. Wittig-Silva C, Whiting M, Lamoureux E, et al. A randomized controlled trial of corneal collagen cross-linking in progressive keratoconus: preliminary results. J Refract Surg. 2008 Sep;24(7):S720-5.
10. Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007 May;26(4):385-9.
11. Mazzotta C, Balestrazzi A, Baiocchi S, et al. Stromal haze after combined riboflavin-UVA corneal collagen cross-linking in keratoconus: in vivo confocal microscopic evaluation. Clin Experiment Ophthalmol. 2007 Aug;35(6):580-2.
12. Raiskup F, Hoyer A, Spoerl E. Permanent corneal haze after riboflavin-UVA-induced cross-linking in keratoconus. J Refract Surg. 2009 Sep;25(9):S824-8.
13. Koller T, Mrochen M, Seiler T. Complications and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009 Aug;35(8):1358-62.
14. Artificial Cornea—The Boston Keratoprosthesis. Massachusetts Eye and Ear. 2012 Jun. Available at:
www.masseyeandear.org/specialties/ophthalmology/cornea-and-refractive-surgery/keratoprosthesis. Accessed July 2012.
15. AlphaCor product overview. Addition Technology. 2012 Jun. Available at:
www.alphacor-ati.com/AlphaCor/index.html. Accessed July 2012.
16. Dokey A, Ramulu PY, Utine CA, et al. Chronic hypotony associated with the Boston type 1 keratoprosthesis. Am J Ophthalmol. 2012 Aug;154(2):266-71.
17. Al-Amri AM, Al-Rashaed S, Al-Kharashi S. Acute retinal necrosis after Boston type 1 keratoprosthesis. Middle East Afr J Ophthalmol. 2012 Apr;19(2):254-7.
18. Panarelli JF, Ko A, Sidoti PA, et al. Angle closure after Boston keratoprosthesis. J Glaucoma. 2012 May 16. [Epub ahead of print]


