Surgeons perform corneal graft surgery for a wide variety of indications, including stromal opacification, corneal ectasias and persistent corneal edema due to endothelial failure. Worldwide, four-and-a-half million individuals have moderate to severe vision impairment secondary to the loss of corneal clarity and more than 200 million are visually impaired.1 Corneal disease is the fifth leading cause of blindness after cataract, uncorrected refractive error, glaucoma and macular degeneration.1 Here we review common graft indications in the United States, clinical pearls for a timely and correct diagnosis and recommendations on when to obtain an initial surgical consult for keratoplasty.
Common Indications and Procedures
The Eye Bank Association of America’s (EBAA) 2016 report revealed that the most common indication for any corneal graft surgery in the US is endothelial cell failure secondary to either Fuchs’ endothelial dystrophy or cataract surgery [e.g., pseudophakic bullous keratopathy (PBK)]. Be aware that a significant portion of PBK patients also have undiagnosed Fuchs’ endothelial dystrophy.2 Therefore, a detailed case history and clinical exam are necessary before referring patients for cataract surgery. The next two most common pathologies for corneal graft surgery are repeat graft surgery after failure and keratoconus.3
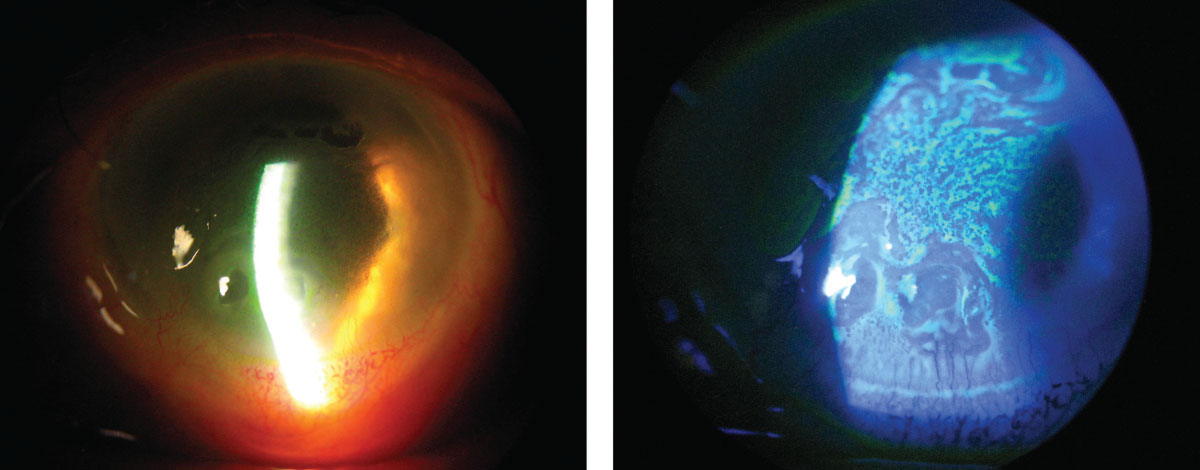 |
| At left, this patient has severe corneal edema associated with complicated cataract surgery. At right, the same patient’s corneal epithelium and irregularity can be easily seen with the sodium fluorescein dye pattern. Click image to enlarge. |
Currently, the United States has the highest rate of corneal transplants per capita.4 In the 2016 EBAA report, endothelial keratoplasty (EK) compromised 57% of surgeries performed in the United States, and full-thickness penetrating keratoplasty (PK) was performed in 38% of patients.3 Anterior lamellar keratoplasty (ALK) and deep anterior lamellar keratoplasty (DALK) procedures accounted for the small portion of remaining surgeries.
Since EK’s introduction in 1999, there has been an impressive growth in the number of surgeries and literature publications, supplanting PK as the mainstay.5,6 In contrast, ALK and DALK surgeries were first introduced in 1959, but their popularity has waned, primarily due to the lack of properly trained ophthalmic surgeons.7 ALK and DALK also require a more prolonged operating room time and carry a high risk of perforation in older patients.
Case History Pearls
Many factors come into play when monitoring patients for corneal edema and considering referral for keratoplasty. Questioning a patient on their visual symptoms is a crucial first step.
• Blurred or variable vision associated with corneal edema often has a diurnal nature—worse upon waking and improved later in the day. This is due to prolonged eyelid closure and a relatively hypotonic tear film, which reverses throughout the day with greater exposure. Symptoms of photophobia, glare, redness, tearing, pain or foreign-body sensation are also commonly associated with corneal edema.
• Most conditions associated with corneal edema present gradually over weeks, months or even years. Symptoms may be so gradual at times that the patient is able to function surprisingly well and at a much higher level than would be expected based on a slit-lamp biomicroscopy exam. Exceptions to this general rule would be edema caused by acutely elevated intraocular pressure (IOP), moderate corneal or intraocular inflammation or hydrops associated with corneal ectasias, such as keratoconus.
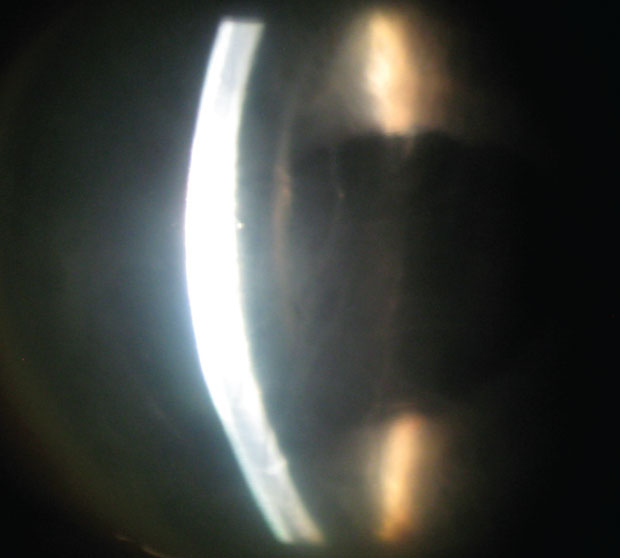 |
| The presence of stromal edema and linear folds of Descemet’s membrane can help you narrow down a diagonsis. |
• Certain factors or special situations can also modify a patient’s quality of vision and affect the surgery referral timeline. Low humidity and modest air movement may lead to visual improvement, whereas endothelial dysfunction from humid days or after a long shower can exacerbate corneal edema. A dehumidifier can be used as a trial and this may even help patients with concurrent ocular allergy. Visual acuity and function may not necessarily correlate well together when considering disabling glare during driving at night or other activities of daily living.
• Clinicians should always obtain a detailed medical history and current list of medications, as inflammatory conditions associated with uveitis (anklosing spondylitis, sarcoidosis) may also cause corneal edema. Amantadine, for example, helps treat Parkinson’s disease and other neurologic diseases but may cause reversible corneal edema if used for a short period of time. Long-term use may cause permanent damage.8,9 Inadvertent exposure of the cornea to topical chlorhexidine preparation, commonly used during cosmetic facial surgery, may predispose a cornea to endothelial failure.10-13
• A number of systemic diseases and medications are associated with corneal opacification. These include metabolic/hereditary disorders such as mucopolysaccharidosis; immune-mediated diseases such as rheumatoid arthritis, Stevens-Johnson syndrome and ocular mucous membrane pemphigoid; and hematologic disorders such as monoclonal gammopathies and malabsorption syndromes, usually following colon resection. Amiodarone, dietary calcium supplementation, periocular radiation and various chemotherapeutic agents have all also been reported as causative agents for opacification.14-17
Examination Pearls
After recording a detailed history, a comprehensive examination of the eye and adnexa is key to determining the etiology of most cases of corneal edema.
• Clinicians should test visual acuity with the lights on and off and compare to produce a qualitative assessment of the individual’s functional status. When performing the external examination, pay careful attention to lid abnormalities, facial asymmetry and evidence of trauma. Slit-lamp biomicroscopy is exceedingly important, especially because it is universally available to ophthalmic providers, and other ancillary testing may not be during initial examination.
• A differential diagnosis of corneal edema or opacification can be narrowed after noting the following: (1) unilateral or bilateral signs, (2) diffuse or localized edema, (3) primarily epithelial or stromal edema, (4) stromal infiltration, striae, thinning, scarring or vascularization and (5) endothelial guttae, Descemet’s tears or detachments, endothelial vesicles, keratic precipitates and peripheral anterior synechiae.
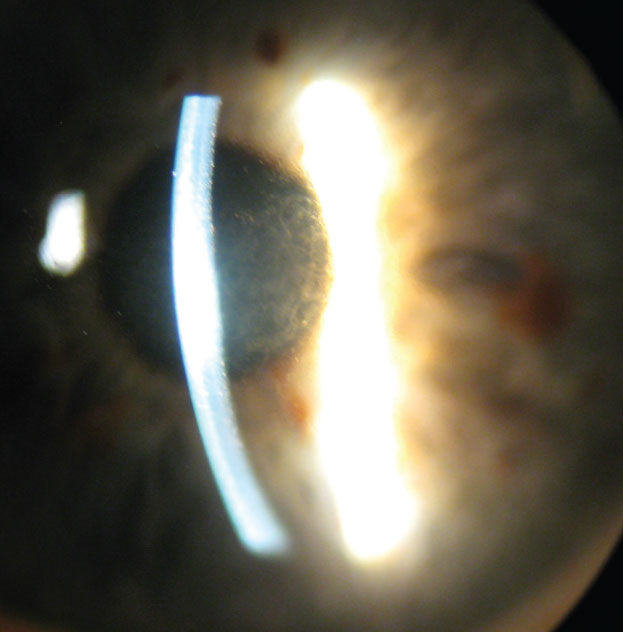 |
| In this patient with Fuchs’ endothelial dystrophy and central stromal edema, note the light dots along the posterior aspect of the corneal light beam. |
• Clinicians can use various slit-lamp techniques such as sclerotic scatter, specular reflection or indirect illumination to evaluate all layers of the cornea. When performing a careful iris examination, note the shape of the pupil, as it is often evidence of past trauma, inflammation or complicated intraocular surgery. IOP measurement by Goldmann applanation tonometry is somewhat unreliable in abnormal corneas, so use alternative devices in concert, such as a pneumatonometer, a Tono-pen device (Reichert) or a dynamic contour tonometer. Consider gonioscopy to rule out retained nuclear fragments, occult foreign bodies and secondary glaucomas that could cause elevated pressure and corneal decompensation.
Ancillary Testing
Other tests can augment the clinical observations from the slit lamp exam.
• A potential acuity meter or pinhole vision using an illuminated near card in a darkened room can assess potential acuity in an effort to bypass anterior segment pathology.
• Disruption of the central or paracentral ocular surface due to corneal edema or scarring can have a surprising impact on vision. Obtain best-corrected vision with spectacles and a rigid gas permeable (RGP) contact lens over-refraction. This can quickly be done in-office by obtaining keratometry measurements and then fitting an RGP lens slightly flatter than the average keratometry reading. Be sure to note the mire pattern to correlate directly with the amount of anterior surface irregularity.
• Corneal pachymetry has become more readily available in ophthalmic offices and is important for quantifying corneal thickness and change over time. Ultrasonic pachymeters provide information about a single spot on the cornea, and their range is often limited to between 200µm and 1,000µm. With careful positioning and probe angulation, an interobserver standard deviation of 12µm is the best to be expected.18
• If precision and peripheral measurements are important, anterior segment optical coherence tomography (AS-OCT) and Scheimpflug imaging systems provide greater accuracy. Since both of these techniques use light instead of sound, each one’s accuracy decreases as stromal opacification worsens.
• The ultrasound biomicroscope provides the most accurate measurements when significant stromal edema is present. Keep in mind that measurements with different devices are not directly comparable in clinical practice; however, most large studies report AS-OCT pachymetry measurements to be lower than ultrasound by between 7µm and 26µm.19,20
• Scheimpflug imaging systems can assess the topographic characteristics of both the anterior and posterior corneal surfaces in addition to corneal thickness measurements. These systems have improved the ability to diagnose forme fruste keratoconus and other corneal ectasias immensely. They can also assess the depth of corneal opacification, which provides useful information for surgical planning.
• AS-OCT provides high resolution, cross-sectional images of the cornea, angle and anterior chamber. Measurement tools to document and follow changes in corneal thickness, angle and anterior chamber are standard with all models. Currently, AS-OCT has the greatest utility for imaging deep and retrocorneal structures, such as a large detached Descemet’s membrane and a retrocorneal membrane. These conditions may arise with trauma, after EK surgery or acute corneal edema associated with keratoconic hydrops. Ultrasound biomicroscopy utility is similar to AS-OCT but is more useful when dealing with extremely opaque corneas and imaging deeper anterior segment structures.
• Endothelial cell count (ECC) is comparable using both specular and confocal microscopy.21 Confocal microscopy provides an advantage over specular when imaging the corneal endothelium in cases of corneal edema. This is particularly useful in cases of unilateral corneal edema where the preoperative diagnosis may be undetermined. Special cases like iridocorneal endothelial syndrome or posterior polymorphous corneal dystrophy have distinctive confocal appearances.
Management
Treatment for corneal edema or opacification may be optical, medical, surgical or a combination, depending on the etiology, nature and severity of the opacity. The needs, desires and health status of the patient also play a part.
The medical agents used to control corneal edema include all ocular hypotensive agents except two drug classes. Prostaglandin analogs should be avoided in patients for whom inflammation is a possible contributing factor—any history of graft rejection or uveitis.22 Also, when endothelial disease is a possible contributing factor, avoid carbonic anhydrase inhibitors as the first line of therapy because of their potential to interfere with the endothelial pump.23 Use topical corticosteroids when inflammation is present and infection has been ruled out. Although hyperosmotic agents or hairdryers are commonly suggested treatment routines, there are no studies that have determined the optimal routines of either of these modalities.
Topical antibiotics and therapeutic contact lenses can be used in conjunction with bullae rupture in PBK to reduce the risk of infection and help control discomfort or pain. Although many different lenses may be used, thin lenses with high water content and a high Dk are thought to have the greatest advantages.24 Ideally, use bandage contact lenses for a finite treatment period; however, longer-term use may be required in many cases, with periodic exchange of the lens.
Patients with corneal edema and persistent discomfort but limited visual potential are better candidates for a conjunctival flap, amniotic membrane or one of many scarification procedures. While less common, a patient with good visual potential may opt for one of these procedures when extenuating circumstances affecting general health or follow-up care/transportation become issues.
Timely referral of patients with endothelial dysfunction is extremely important because patients with chronic PBK often develop a layer of subepithelial fibrosis. If a patient already has considerable subepithelial scarring, this will prevent a good visual outcome following an EK. They would instead need full-thickness PK or a superficial keratectomy combined with EK.
For patients with endothelial dysfunction, it is important to address considerations beyond visual acuity assessment. EK as a combined procedure should be considered in the context of a visually significant cataract and even early cataracts with the presence of corneal edema. Ultrasound pachymetry measurement in excess of 640µm or the presence of epithelial edema suggest the cornea may decompensate with intraocular surgery.25
An ECC below 800 has also been suggested as predictive of poor endothelial cell function post-cataract surgery. Confluence of guttae, even in the absence of stromal or epithelial edema, can reduce visual acuity, contrast sensitivity and increase symptomatic glare for patients.25 Consider these criteria along with activities of daily living to prompt an early referral for EK. Once subepithelial scarring has occurred with chronic PBK, an early referral is less important, since PK will be the preferred surgical technique.
Prior to 2000, virtually all corneal transplant candidates with decompensated corneas underwent PK. However, according to the 2011 EBAA report, approximately 75% of patients with corneal edema are now managed with EK.26 This broad acceptance of EK is due to its rapid visual recovery, its significantly greater optical (both astigmatic and refractive) predictability, the presence of greater wound strength and its lesser risk for rejection with a greater long-term survival rate.26
For patients with corneal subepithelial or stromal scarring, the procedure of choice will be PK, and, therefore, early consultation for graft surgery is less important. These patients may benefit more from initially improving ocular surface health (addressing tear film stability, lid function and hygiene), controlling IOP in the setting of glaucoma and stabilizing glycemic control in diabetic populations.
As corneal graft surgery evolves, so do our pre-op considerations. New surgical techniques make decisions regarding your patient all the more important, as each case may do better with one procedure over another. Practitioners must remain vigilant when observing patients for signs of edema and know when the time is right to refer them for keratoplasty.
Dr. Esposito is an attending provider and clinical researcher at the New Mexico Veterans Administration Health Care System Eye Clinic in Albuquerque, New Mexico. He is an adjunct clinical professor at the University of Houston College of Optometry, the Pacific University College of Optometry and the New England College of Optometry.
|
1. Flaxman SR, Bourne RRA, Resnikoff S. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221-34. 2. Eye Bank Association of America. 2016 Eye Banking Statistical Report. Washington DC: Eye Bank Association of America; 2017. 3. Afshari NA, Pittard AB, Siddiqui A. Clinical Study of Fuchs corneal endothelial dystrophy leading to penetrating keratoplasty: a 30-year experience. Arch Ophthalmol. 2006;124:777-80. 4. Gain P, Jullienne R, He Z. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134:167-73. 5. Melles GR, Lander F, Beekhuis WH. Posterior lamellar keratoplasty for a case of pseudophakic bullous keratopathy. Am J Ophthalmol. 1999;127:340-1. 6. Terry MA, Ousley PJ. Endothelial replacement without surface corneal incisions or sutures: topography of the deep lamellar endothelial keratoplasty procedure. Cornea. 2001;20:14-8. 7. Hallermann W. Some remarks on keratoplasty. Klin Monbl Augenheilkd Augenarztl Fortbild. 1959;135:252-9. 8. Jeng BH, Galor A, Lee MS. Amantadine-associated corneal edema potentially irreversible even after cessation of the medication. Ophthalmology. 2008;115:1540-4. 9. Naumann GO, Schlotzer-Schrehardt U. Amantadine-associated corneal edema. Ophthalmology. 2009;116:1230-1. 10. Phinney RB, Mondino BJ, Hofbauer JD. Corneal edema related to accidental Hibiclens exposure. Am J Ophthalmol. 1988;106:210-5. 11. Van Rij G, Beekhuis VH, Eggink CA, et al. Toxic keratopathy due to the accidental use of chlorhexidine, cetrimide and cialit. Doc Ophthalmol. 1995;90:7-14. 12. Ohguro N, Matsuda M, Kinoshita S. The effects of denatured sodium hyaluronate on the corneal endothelium in cats. Am J Ophthalmol. 1991;112:424-30. 13. Varley GA, Meisler DM, Benes SC. Hibiclens keratopathy: a clinicopathologic case report. Cornea. 1990;9:341-6. 14. Kaplan LJ, Cappaert WE. Amiodarone keratopathy. Correlation to dosage and duration. Arch Ophthalmol. 1982;100:601-2. 15. Jhanji V, Rapuano CJ, Vajpayee RB. Corneal calcific band keratopathy. Curr Opin Ophthalmol. 2011;22:283-9. 16. Jeganathan VS, Wirth A, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiat Oncol Biol Phys. 2011;79:650-9. 17. Van Meter WS. Central corneal opacification resulting from recent chemotherapy in corneal donors. Trans Am Ophthalmol Soc. 2007;105:207-13. 18. Miglior S, Albe E, Guareschi M. Intraobserver and interobserver reproducibility in the evalutation of ultrasonic pachymetry measurements of central corneal thickness. Br J Ophthalmol 2004;88:174-7. 19. Wirbelauer C, Scholz C, Hoerauf H. Noncontact corneal pachymetry with slit lamp-adapted optical coherence tomography. Am J Ophthalmol. 2002;133:444-50. 20. Zhao PS, Wong TY, Wong WL. Comparison of central corneal thickness measurements by visante anterior segment optical coherence tomography with ultrasound pachymetry. Am J Ophthalmol 2007;143:1047-9. 21. Mustonen RK, McDonald MB, Srivannaboon S, et al. In vivo confocal microcsopy of Fuchs’ endothelial dystrophy. Cornea. 1998;17:493-503. 22. Aydin S, Ozcura F. Corneal edema and acute anterior uveitis after two doses of travoprost. Acta Ophthalmol Scand 2007;85:693-4. 23. Wirtitsch MG, Findl O, Heinzl H, Drexler W. Effect of dorzolamide hydrochloride on central corneal thickness in humans with corneal guttata. Arch Ophthalmol. 2007;125:1345-50. 24. Foulks GN, Harvey T, Raj CV. Therapeutic contact lenses: the role of high-Dk lenses. Ophthalmol Clin North Am 2003;16:455-61. 25. Seitzman GD, Gottsch JD, Stark WJ. Cataract surgery in patients with Fuchs’ corneal dystrophy: expanding recommendations for cataract surgery without simultaneous keratoplasty. Ophthalmology. 2005;112:441-6. 26. Wu El, Ritterband DC, Yu G. Graft rejection following descemet stripping automated endothelial keratoplasty: features, risk factors, and outcomes. Am J Ophthalmol. 2012;153:949-57. |


