While sex differences may not matter for many things in eye care, they can have a significant impact on dry eye disease (DED), according to the Tear Film and Ocular Surface Society’s (TFOS) Dry Eye Workshop II (DEWS II) report.1 In addition to sex, hormones and gender have also been identified as factors that can affect DED diagnosis and treatment.
In the absence of a decent comprehensive report discussing the biological sex differences at a cellular and molecular level, TFOS commissioned one by the Institute of Medicine to help develop a better understanding.2 The report documents several biological differences between the sexes that manifest themselves in the anatomy, physiology and pathophysiology of the lacrimal gland, cornea, conjunctiva, meibomian glands, nasolacrimal duct and tear film.
In addition, men and women experience pain differently, which impacts the number of patients seeking care for DED and experiencing symptom improvement. Studies show that men have a higher pain tolerance than women, with explanations ranging from sociocultural gender roles to brain neurochemistry differences.3,4 Thus, male patients are less likely to complain of DED symptoms and are less likely to seek treatment or be compliant with treatment. When they do, they tend to have a greater decrease in symptoms following LipiFlow (TearScience) treatment.1 Alternately, females may present with symptom complaints that may not match their ocular signs.
Here’s how sex, gender and hormones affect a patient’s risk for, and diagnosis of, dry eye.
Work With the Right Definitions |
Risky Behavior
Overall, the female sex has a higher risk of DED, and women are diagnosed an average of six years younger than their male counterparts.1,5-8
Additionally, certain gender-related behaviors and habits can lead to a higher risk of DED, such as the use of cosmetics and contact lens wear, which is higher in women than men. Women are also more likely to undergo LASIK refractive surgery, which carries an elevated risk of post-op DED and neuropathic pain. Animal studies show females have a higher prevalence of neuropathic pain related to DED.1 Gender- and sex-specific medications, such as hormone replacement therapy and oral contraceptives, can also increase the risk of DED.
Signs & Symptoms See-Saw
The female sex reports more severe symptoms as measured by the Ocular Surface Disease Index and the Symptom Assessment Questionnaire in Dry Eye. Increased visual quality indicators and feelings of depression accompany this increase in symptoms.1 The increase in reporting and scoring of symptoms by females may, in part, be why they are diagnosed an average of six years younger than male patients.1
Tear osmolarity testing has become common in many optometric offices and can aid in diagnosing and managing DED. The DEWS II report noted that both males and females experience increased tear osmolarity during aging.1 Incorporating tear osmolarity testing in all older patients may lead to increased and earlier diagnosis of DED patients who may otherwise not have been identified.
Neutral lipids are unstable when spread over an aqueous subphase and will collapse, forming lipid droplets that leave the aqueous portion of the tear film unprotected, allowing for rapid evaporation.9,10 This layer is stabilized by the lower polar lipid sublayer, so too few polar or too many neutral lipids can cause a tear film imbalance.9,10 DEWS II researchers note that meibomian gland expression of specific polar lipids in meibum was lower in both middle aged males and females.1 Specific neutral lipid expression in meibum was higher in older males and females.1 Both of these results correlate with the finding that the lipid layer is thicker and less contaminated in males older than age 45.1 It also explains, in part, why in males in the third decade of life experience their highest rate of decreased tear break-up time and females in their seventh.1
Within the last few years, more clinicians have started to use meibography to assess patient’s meibomian gland function and structure. The TFOS researchers behind DEWS II found that although males have a higher prevalence of asymptomatic meibomian gland dysfuction (MGD), they have greater lid margin abnormality and gland drop out after age 70.1 This may explain why males have greater decrease in symptoms following LipiFlow treatment.1
Goblet cells found within the conjunctiva are important because they produce and secrete mucins, which in turn hydrate and lubricate the surface of the eye, helping to control chronic inflmmation.11 Mucin regulation is targeted by allergy and inflammatory mediators that alter the function and survival of the goblet cells.11 Allergic conjunctivitis leads to an increase in mucin production, while the opposite occurs with DED.11 With chronic inflammation, inflammatory cytokines cause decreased goblet cell survival, leading to decreased mucin production.11
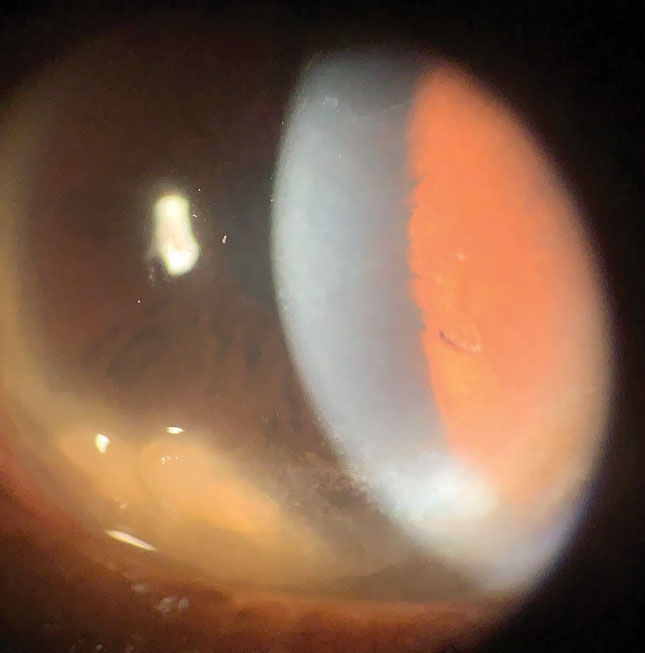 |
| This postmenopausal female patient has chronic dry eye and bilateral Salzmann’s nodules. Click images to enlarge. |
 |
The DEWS II report notes that males have higher goblet cell count, suggesting males may fare better overall when it comes to goblet cell density loss even when compromised by chronic inflammation. Males’ higher goblet cell count is also likely related to their higher prevalence of conjunctival inflammation related to allergies.1
DEWS II also found women experience a depression of goblet cell count around the time of ovulation.1 Knowing that females who are ovulating will have cyclically worse dry eye, it may be helpful to individualize their DED treatment and increase their use of medication around their mensturation cycle.
Goblet cell density and production is also affected by other inflammatory processes such as superior limbic keratoconjunctivitis, which is more prevalent in women.1
Pterygia, which originate in the conjunctiva and extend onto the cornea, exhibit areas of goblet cell hyperplasia and can occur from many things, such as ultraviolet radiation, inflammatory disorders and chronic ocular surface irritatiton.12 The DEWS II report finds that pterygia occur more often in males but cause greater discomfort in females.1 It is important to keep this in mind when treating pterygia and making surgical decisions. Even if male patients may be asymptomatic, don’t forego the discussion of the risks of progression and don’t delay treatment of underlying causes.
Comorbidities, although widely reported in multiple studies to increase the risk of DED, have not been directly studied when it comes to sex-related differences. Research shows lupus, Sjögren’s syndrome, rosacea, anxiety, hay fever, depression, pelvic pain, irritable bowel syndrome and chronic pain syndrome are all associated with DED.1,5 Clinically, most agree with this and have noted increased DED incidence in patients who suffer from these comorbidities. Worth noting is that an all-male study also found an increased rate of DED in those exhibiting high blood pressure and benign prostatic hyperplasia.6 These conditions are not typically linked with DED, and we may be overlooking these patients.
Both sexes have an increased risk of DED with the use of antidepressants, although women are more likely to use them. Not surprisingly, women who are postmenopausal and on hormone therapy had a 70% increased incidence of DED when on estrogen alone and a 30% increased incidence when on estrogen in combination with progesterone/progestins.5
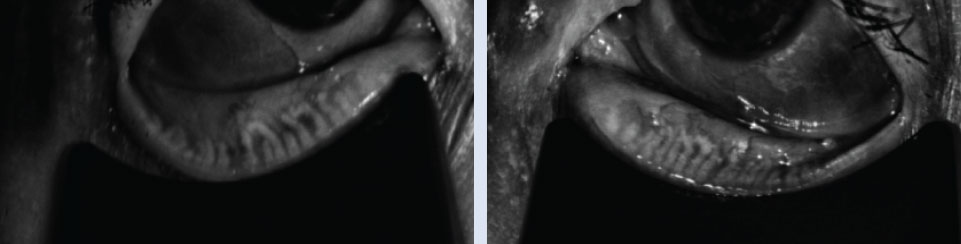 |
| Right and left meibography of a 75-year-old female patient with no diagnosed systemic comorbidities. Click image to enlarge. |
Get To know Hormones
Our endocrine system helps us develop and regulate the ocular surface and adnexa. Hormones play an important role in this system, including androgen, estrogen, progestin, estrogen, glucocorticoids, growth hormone IGF-1, insulin and thyroid hormones.
Androgen. This hormone helps in the development and function of the lacrimal glands, meibomian glands, cornea and conjunctiva. The lacrimal gland is an androgen target organ that uses the hormone to help with cellular architecture, gene expression, protein synthesis, immune activity and fluid and protein secretion.1
Shortage can cause lacrimal gland dysfunction and aqueous tear deficiency. Women have a decrease in serum androgen and increased primary lacrimal gland deficiency during menopause, pregnancy, lactation and with use of estrogen containing oral contraceptives.
Sjögren’s patients, more commonly women than men, have a greater androgen deficiency within their lacrimal tissues related to the elevated pro-inflammatory cytokines IL-1, TNF-α and IL-6.1 This androgen deficiency impairs lacrimal gland function, which is a risk factor for lacrimal gland inflammation and aqueous-deficient DED.1
The meibomian gland complex is also an androgen target organ, stimulating tissue function and suppressing keratinization.1 Androgen stimulates the ontologies responsible for lipid biosynthesis and cholesterol, fatty acid, phospholipid and steroid dynamics.1 Androgen deficiency is a risk factor for the development of MGD and evaporative DED.
Androgen is also important for corneal and conjunctival cell reproduction and synthesis. Deficiency can lead to poor wound healing, corneal dystrophies and increased conjunctival and corneal staining.1 Studies exploring topical and systemic applications show an improvement in DED signs and symptoms in both men and women.1
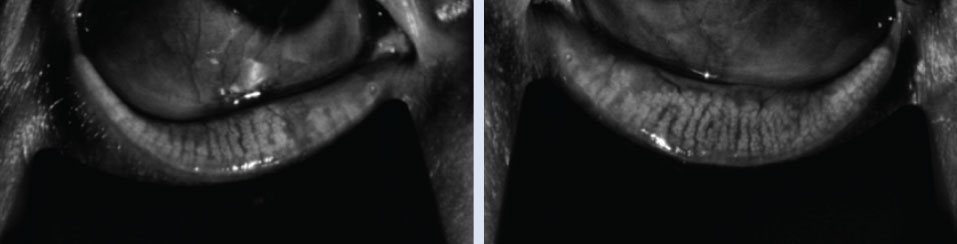 |
| Right and left meibography of a 75-year-old male patient with no diagnosed systemic comorbidities. Note the male patient of the same age has less gland damage, truncation and atrophy. Click image to enlarge. |
Estrogen and progesterone. Unlike androgen, less is understood about the roles of estrogen or progesterone in ocular surface function. No intra-tissue data currently exists; however, both hormones have been detected in human tears, correlating with serum levels in premenopausal females.1 Estrogen receptors are found in the meibomian glands, lacrimal gland, cornea, bulbar conjunctival and tarsal plate.1
Estrogen plays an important role within our immune system, the extent of which differs significantly based on concentration and tissue type. This hormone promotes the production of B-cells and antibodies, a subset of T-cells, dendritic cells, M2 macrophages and regulatory cytokines.1
Reports on the effect of estrogen and progesterone on DED are varied depending on the study and co-factors such as autoimmune conditions.1 Although studies show positive effects of topical testosterone on MGD and DED, it is currently only available via compounding pharmacies.9 Allergan submitted a patent and initiated a Phase II study in 2015, but nothing has been published at this time. Similarly, compounded estradiol suspension or a mixture containing testosterone and progesterone are used by some practitioners to treat DED off label.
Glucocorticoids. Functioning as a regulator of the inflammatory response and immunosuppressive hormone, glucocorticoid and its synthetic partners are important in anti-inflammatory activity.
Glucocorticoid’s effects on the ocular surface and adnexa are concentration dependent and have a higher level of anti-inflammatory activity in males than females.1 The ocular surface of the mucosa is immunoprotected in part by the autocrine effect in epithelial cell and fibroblasts caused by production of cortisol from endogenous glucocorticoid.1
With a better understanding of these hormones’ role in DED, researchers have developed and continue to develop new treatments. Topical glucocorticoids are already widely used to control inflammation-associated DED. A phase IV study recently finished, but has not published results, on topical hydrocortisone 0.335% (Softacort, Thea Pharmaceuticals) to treat chronic DED and ocular inflammation. Another ongoing study is looking at the effects of intravenous glucocorticoid on the tear film in eyes with thyroid-associated ophthalmopathy.
With the currently available topical steroids, clinicians should take the preservatives within the drug into consideration, as many with DED are also sensitive to these. Compounding pharmacies are a great alternative for preparation of preservative-free topical corticosteroids. This option also allows for control of the drug’s concentration, helping to decrease concern for complications with longer-term use.
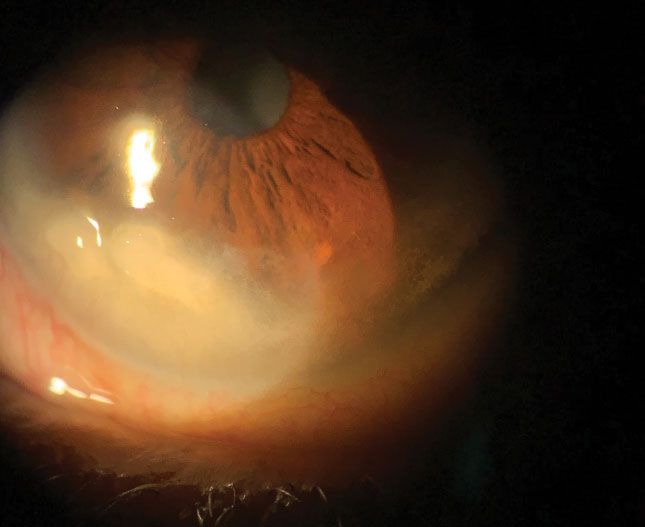
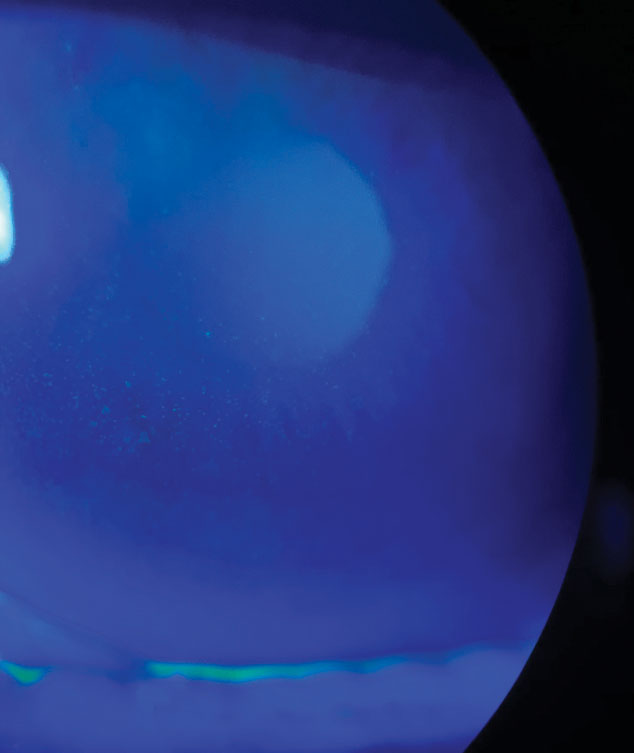 |
| This female patient reported severe symptoms, despite signs of only 1+ superficial punctate keratitis. Click image to enlarge. |
Growth hormone, IGF-1 and Insulin. The latter of these hormones, secreted by the acinar cells within the lacrimal gland, is found within the human tear film and its receptor, IGF-1, is found on the human ocular surface. Growth hormone and IGF-1 may help within the meibomian glands to regulate growth and function.1
Insulin helps with corneal wound healing by promoting tissue maintenance.1 Diabetic patients have delayed corneal healing in part because their tears exhibit increased IGF-binding protein 3, which may attenuate the IGF-1 receptor signal necessary for tissue maintenance.
Diabetes patients often suffer from DED due to the autoimmune destruction of the lacrimal gland from antigen cross activity with the pancreas in Type 1 diabetes. DED in patients with Type 2 diabetes is both hormonal and metabolic in nature stemming from the defective insulin action and hyperglycemia.
Sex hormones are thought to influence the levels of insulin receptor, suggesting higher levels of sex hormones lower the action of insulin within ocular tissues. Patients with insulin resistant conditions such as polycystic ovary syndrome, pregnancy, anti-androgen therapy and androgen insensitivity syndrome have increased DED symptoms.
Animal studies looking at systemic replacement or topical insulin treatment show that signs of DED and wound healing defects can be reversed. Current use of autologous serum, which contains insulin and growth factors, is an effective treatment for severe DED and conditions associated with it, although it is widely underused due to preparation and storage inconveniences.
Thyroid. An imbalance of thyroid hormones T3 and T4 has a negative effect on the lacrimal gland, tear film and ocular surface.1 These hormones help to promote lipid and protein synthesis along with tissue growth.1 Females are more prone to thyroid diseases such as Hashimoto’s thyroiditis and Graves’ disease. These patients are also more likely to have Sjögren’s syndrome.
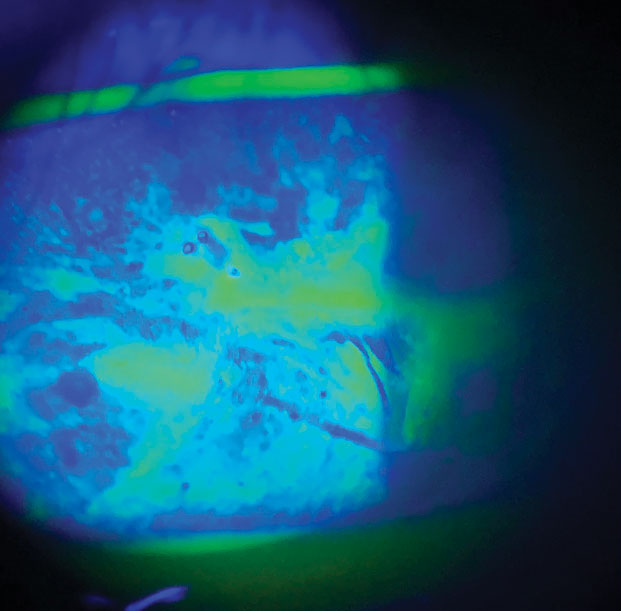 |
| This poorly controlled diabetic patient is treated for chronic severe DED and neurotrophic keratoconjunctivitis, which has led to recurrent corneal abrasions. Click image to enlarge. |
Our understanding of DED has evolved significantly in the last 10 years, as has our diagnostic technology and treatment options. Understanding the underlying cause and how sex and gender impact the physical, hormonal and behavioral differences are crucial to ensure an appropriate treatment approach.
Dr. Koetting is the referral optometric care and externship program coordinator at Virginia Eye Consultants in Norfolk, VA. She is a fellow of the AAO and a trustee of the Virginia Optometric Association.
1. Sullivan D, Rocha E, Aragona P, et al. TFOS DEWS II sex, gender, and hormones report. Ocul Surf. 2017;15(3):284-333. 2. Institute of Medicine (US) Committee on Understanding the Biology of Sex and Gender Differences: Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, DC: The National Academies Press; 2001. 3. Mogil, Jeffrey S. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859. 4. Ruau D, Liu LY, Clark JD, et al. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012;13(3):228-34. 5. Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318-26. 6. Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol 2009;127:763e8. 7. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806. 8. Muoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: The Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118(6):819-25. 9. Green-Church K, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52(4):1979-93. 10. Shrestha R, Borchman D, Foulks G, et al. Analysis of the composition of lipid in human meibum from normal infants, children, adolescents, adults, and adults with meibomian gland dysfunction using 1H-NMR spectroscopy. Invest Ophthalmol Vis Sci. 2011;52(10):7350-8. 11. Dartt DA, Masli S. Conjunctival epithelial and goblet cell function in chronic inflammation and ocular allergic inflammation. Curr Opin Allergy Clin Immunol. 2014;14(5):464-70. 12. Golu T, Mogoantă L, Streba CT, et al. Pterygium: histological and immunohistochemical spects. Rom J Morphol Ebryol. 2011;52(1):153-8. |


