 |
The Epithelium in Distress: From RCE to Dystrophy
To provide comprehensive patient care, optometrists must have a clear understanding of the function and pathophysiology of this structure.
By Karen K. Yeung, OD, and Rachel Snyder
Release Date: February 15, 2023
Expiration Date: February 15, 2026
Estimated Time to Complete Activity: 2 hours
Jointly provided by Postgraduate Institute for Medicine (PIM) and Review Education Group.
Educational Objectives: After completing this activity, the participant should be better able to:
Recognize the pathophysiology of this structure.
Describe a healthy epithelium and how it functions.
Discuss the various conditions that can impact the epithelium.
Identify when a patient’s epithelium is in distress.
Target Audience: This activity is intended for optometrists engaged in managing patients with epithelial damage.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by PIM and the Review Education Group. PIM is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education and the American Nurses Credentialing Center to provide CE for the healthcare team. PIM is accredited by COPE to provide CE to optometrists.
Reviewed by: Salus University, Elkins Park, PA
Faculty/Editorial Board: Karen Yeung, OD, and Rachel Snyder
Credit Statement: This course is COPE approved for 2 hours of CE credit. Activity #125344 and course ID 82691-GO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements: PIM requires faculty, planners and others in control of educational content to disclose all their financial relationships with ineligible companies. All identified conflicts of interest are thoroughly vetted and mitigated according to PIM policy. PIM is committed to providing its learners with high-quality, accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Those involved reported the following relevant financial relationships with ineligible entities related to the educational content of this CE activity: Authors—Dr. Yeung and Ms. Snyder have no financial interests to disclose. Managers and Editorial Staff—The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
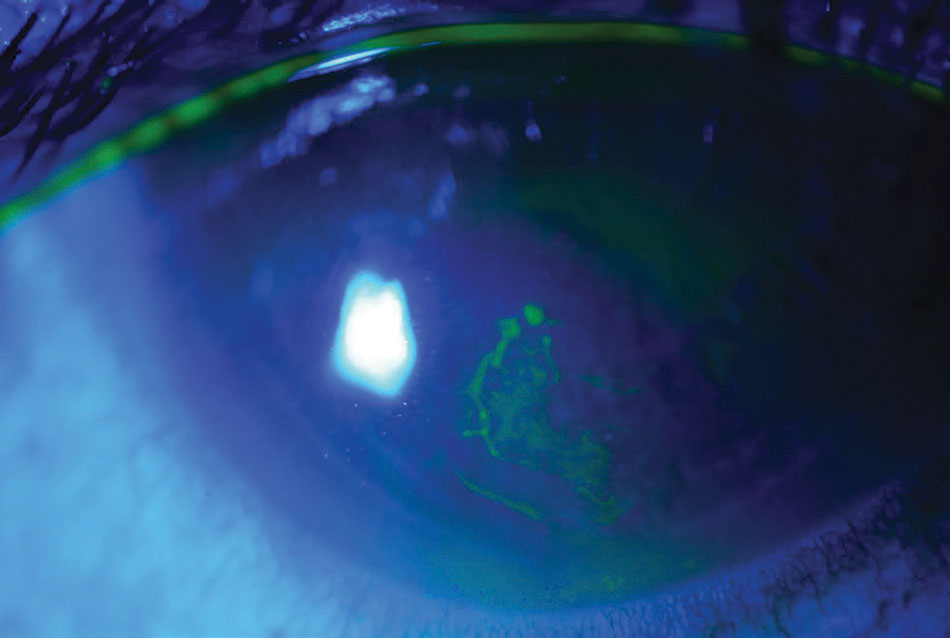 |
Pictured in this eye is an RCE. Photo: Rebecca Rojas, OD. Click image to enlarge. |
The corneal epithelium, which is a mechanical, immunological and biological barrier to the outer environment, is only five to seven uniform cell layers deep and 50µm thick. Disruption of the epithelium from injuries, dry eyes, allergies, infectious and noninfectious entities and corneal dystrophies can lead to clinical diseases that compromise the health of the entire cornea. Fortunately, damage to the corneal epithelium, as long as it does not extend below Bowman’s layer, will not cause scarring or permanent visual loss once resolved.
This article will highlight the various issues that can arise—from recurrent corneal erosions (RCEs) to dystrophies—and lead to epithelial stress and possibly vision loss. Though many ocular conditions penetrate deeper than the epithelium, only the pathophysiological effects on this structure will be discussed.
Epithelial Structure
The epithelium is comprised of three layers: apical cells, wing cells and basal cells. The corneal epithelium undergoes involution, apoptosis and desquamation every seven to 10 days, which plays a large role in shedding infected cells from the corneal surface.1
Apical cells. This superficial aspect is two to three layers of flat polygonal, non-keratinized squamous cells. On their anterior surface are microplicae and microvilli, which extend up to 0.5µm into the tear film, hence increasing the surface area. Coated with a dense glycocalyx, apical cells help stabilize the surface tear film. Desmosomes form tight junctions between the superficial layer cells, creating a selective barrier to substances in the tear film.2
Wing cells. Below the superficial layer are two to three layers of wing cells that adhere together by desmosomes and adherens junctions. They have gap junctions that allow molecules to be exchanged directly between cells.
Basal cells. Below the wing cells is a single layer of columnar cells forming the basal epithelium, which adheres the epithelium to Bowman’s membrane. They are the source of wing and apical cells, since they are the only corneal epithelial cells that are capable of mitosis. The corneal epithelium self-regenerates, turning over all of the cells in approximately five to seven days.
Corneal Abrasions, RCEs
Superficial abrasions in the cornea can be caused by trauma, foreign body insult, including contact lenses (CLs), or spontaneously. They are common across all age groups and responsible for 3% of eye concerns in primary care clinics.3 Scarring of the cornea will not occur unless the abrasion reaches below Bowman’s layer. Upon slit lamp evaluation, topical positive fluorescein stains any irregular, loose or missing epithelium from an abrasion. Any negative staining represents elevations on the epithelium.
Corneal abrasions, sometimes years later, can abrade again as RCEs. In fact, 45% to 64% of RCEs occur after corneal trauma to the superficial cornea. The next most common cause of RCE is epithelial basement membrane dystrophy (EBMD), with 19% to 29% of these patients having RCE.4 The highest prevalence of EBMD is in adults between 30 and 40 years old; however, it also affects those between 30 and 80 years of age.5 Furthermore, there is also a high rate of RCE in individuals with dry eyes, diabetes, blepharitis, ocular rosacea, other corneal epithelial dystrophies and corneal degenerations.5,6
RCE is associated with a sudden onset of pain, especially upon wakening. Ocular desiccation from sleeping with the eyelids closed causes adhesions between the tarsal conjunctiva and corneal epithelium. When the eye opens, the shearing force of the eyelids avulses the corneal epithelium from the epithelial basement membrane due to the weakened or dysfunctional hemidesmosome attachment between the epithelium and the basement membrane.5,7 Primarily unilateral (unless accompanied by a dystrophy) symptoms lasting from minutes to hours may include photophobia, redness, blurred vision and tearing. Persistent corneal defects may last for days, though neurotrophic keratitis should be considered in epithelial defects that last 10 or more days even with palliative treatment.8 Slit lamp findings may show conjunctival injection, epithelial defects or corneal signs indicative of a corneal dystrophy (e.g., map-dot-fingerprint for EBMD).
 |
| Click table to enlarge. |
Keratitis
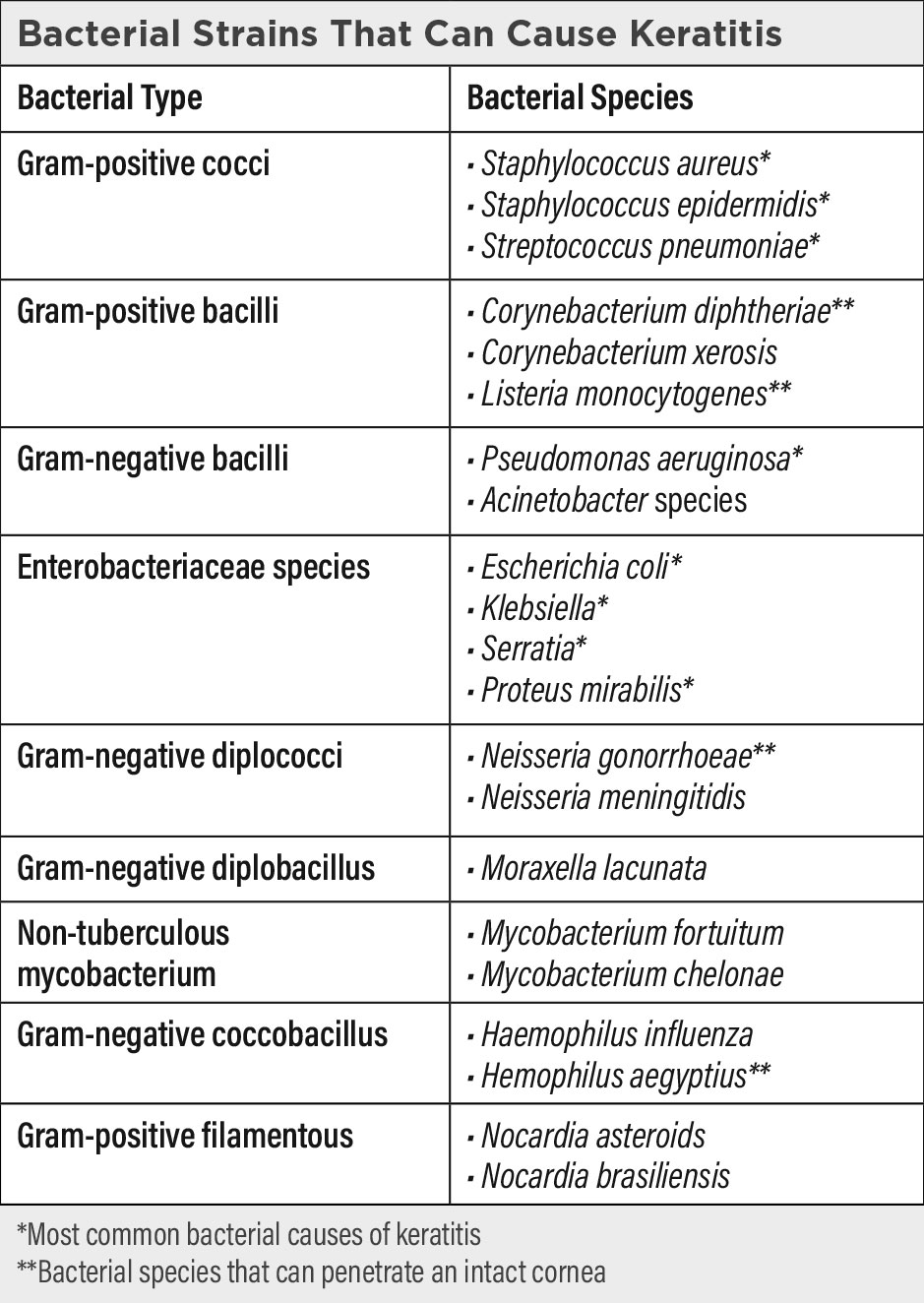
An inflammation of the cornea from both infectious and noninfectious etiologies, keratitis can be localized or systemic. It is generally associated with corneal edema, infiltration of inflammatory cells and ciliary congestion. Noninfectious etiologies include those from dry eyes, allergies and neurotrophic corneal disorders. Infectious organisms include viruses, bacteria, protozoa, parasites, fungi and oomycetes. Most organisms cannot penetrate an intact epithelium, so many infections occur with the presence of epithelial damage or compromise. There are a few extremely virulent organisms, including Neisseria gonorrhea, Haemophilus aegyptius, Corynebacterium diphtheriae, Bacterium diphtheria and Listeria species, that can penetrate an intact corneal epithelium resulting in severe corneal insults.9
Pseudomonas and Staphylococcus are the most common causes of keratitis in CL wearers, followed by Acanthamoeba. Fungal and viral corneal infections are generally not specific complications of lens wear. Factors that contribute to CL-related keratitis include extended wear, overwear, corneal hypoxia, poor lens hygiene, rinsing of CLs in tap water, swimming in lenses, lens-induced corneal trauma and cellular toxicity from multipurpose CL solutions.10,11 The severity of the infection is dependent on the pathogenicity of the microbe, the underlying condition of the cornea and the individual’s immunological state. After breaching the epithelium, the pathological microbes can cause keratitis and invade the stroma.
It is important ODs recognize both noninfectious and infectious etiologies of keratitis. We will first discuss the various noninfectious causes that may present in optometric practice.
Dry eyes. Depending on the population, dry eye can affect 5% to 35% of individuals.12 It can be caused by a myriad of conditions including dry/drafty environments, CLs, eye drop preservative toxicity, prolonged computer use, post-LASIK and systemic medications. The resultant tear hyperosmolarity triggers an inflammatory event that causes the protease-mediated lysis of tight junctions between the epithelial cells and eventual cell apoptosis.13,14 The epithelial surface becomes irregular, and areas of desiccation cause sensitization of epithelial nociceptors. In chronic dry eyes, the areas of desiccation appear as superficial punctate keratitis (SPK) with sodium fluorescein staining. The corneal epithelium becomes thinner as the density of its three layers decrease.15 Epithelial cells also become enlarged and irregularly shaped. Individuals report foreign body sensation with their dry eyes.
Thygeson’s SPK. This condition appears as one to 50 multiple bilateral, intraepithelial, elevated, whitish-gray, granular-looking corneal epithelial lesions in the center of the cornea. Some of the opacities have a raised center that breaks through the epithelial surface as evident with sodium fluorescein staining, which is the main cause of foreign body sensation, photophobia and slightly reduced vision. Thygeson’s SPK is chronic with multiple insidious onsets, long durations without serious sequela and remissions.16 During remissions, the opacities may completely disappear unless there is subepithelial scarring that does not stain with fluorescein.17 The etiology of Thygeson’s remains unknown.
Allergies. Allergic conjunctivitis affects up to 40% of the US population, and 30% of these individuals have corneal involvement.18 Allergies affecting the cornea occur from the loss of barrier function of the corneal epithelium.19 These patients have an altered organization of the tight junctions and abnormal expression of junctional proteins. This allows allergens to cleave the tight junctions between superficial epithelial cells and penetrate the paracellular space, eliciting the strong innate immune response and the chronic inflammation/corneal swelling that generally occurs with allergies. Clinically, symptoms include itchy eyes, foreign body sensation and tearing.
Neurotrophic keratitis. Any ocular or systemic condition that damages the trigeminal nerve (cranial nerve V) or the corneal nerve plexus can cause neurotrophic keratitis. Etiologies may include herpetic keratitis, trigeminal nerve damage caused by orbital or head injury, surgery, strokes, chemical burns, physical injuries, corneal surgeries, chronic medication use and CL wear.20,21 Other causes may be from systemic disease such as diabetes, leprosy, multiple sclerosis and intracranial masses that affect the trigeminal nerve such as aneurysms, meningioma and schwannoma.21
The corneal epithelium is highly innervated, and any decreased corneal nerve sensitivity can result in corneal surface desiccation, SPK, persistent epithelial defects and ulcers that can progress to stromal melting and corneal perforation.21 Animal models have shown that within hours of ocular nerve damage, the apical epithelial cells swell, lose their microvilli surface and slough off at a rapid rate into the tear film.22 The cornea thins, and there is also decreased epithelial wound healing, and the new epithelium is prone to RCEs.23
As previously mentioned, there are also a number of infectious etiologies of keratitis. These are discussed in more detail below.
Viruses. Common viruses that can affect the corneal epithelium include herpes simplex virus (HSV), varicella zoster virus (VZV) and adenovirus. As the first line of defense, corneal epithelial cells initiate the corneal immune response by releasing proinflammatory cytokines and chemokines to recruit mononuclear lymphocytes and neutrophils into the cornea. Epithelial cells also produce interferons to enhance antiviral activities.
While HSV1 involves the cornea through direct contact, HSV2 can be transmitted to the eye through infected venereal secretions. In both HSV1 and 2, the primary infection rarely involves the cornea, but unfortunately this is when the virus is carried to the ophthalmic branch of the trigeminal ganglion and becomes latent. Similarly, VZV’s primary infection causes chickenpox, but following the primary infection it establishes latent infection in neuronal cells in the peripheral ganglia and affects the cornea if the ophthalmic branch is involved. Both HSV and VZV can be latent in the same ganglion.24 Reactivated latent virus can result in corneal infection when the latent virus becomes reactivated, resulting in shedding of the virus on the corneal surface. Reactivation can cause epithelial dendritic or geographic keratitis.
Among adenoviruses, epidemic keratoconjunctivitis (EKC) is the only form of adenovirus that can affect the cornea. However, it is also highly contagious and causes severe keratitis. Adenoviruses are robust, resilient to standard disinfection methods and easily transmitted in high-density populations. They spread via droplets through the respiratory tract or eye or by a contaminated medical device/unclean surface through viral shedding. While most adenovirus infections are self-limiting, the keratitis can persist or recur months to years after the initial infection. EKC presents with punctate or large, geographically-shaped epithelial erosions that typically resolve in several days.25 Unfortunately, 60% of EKC infections will reach the stroma and cause stromal keratitis.26
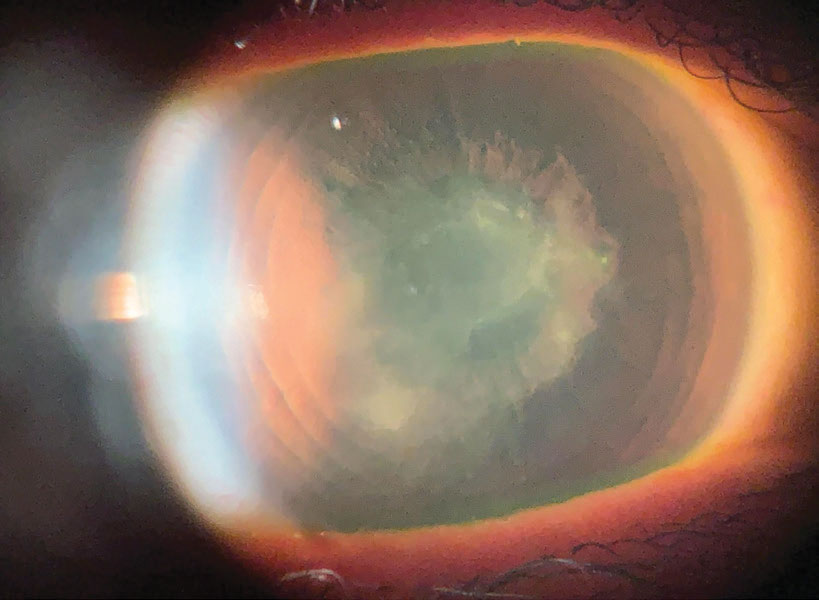 |
A corneal scar from HSV with a persistent epithelial defect after a corneal erosion. Photo: Cecelia Koetting, OD. Click image to enlarge. |
Bacteria. With the increase of CL use over the past few decades, the rate of bacterial keratitis has increased proportionally.27 Fortunately, single-use CLs are becoming more prevalent. With the lowest rate of environmental organism adherence, they have protective effects from infections.28
Bacterial keratitis is a major cause of sight-threatening emergencies once the epithelium is breached.29 A majority of bacterial keratitis cases arise from Staphylococcus aureus, Streptococcus pneumoniae, Pseudomonas aeruginosa and species of the Enterobacteriaceae family (including Klebsiella, Enterobacter, Serratia and Proteus). The ocular pathogenicity of different types of bacteria is related to their ability to adhere to and invade corneal epithelial cells.30 Pseudomonas aeruginosa is the most frequent cause of bacterial keratitis and is also the most pathogenic, with the capability of corneal perforation within 72 hours. The keratitis has a “ground glass” appearance.31
Gram-positive bacteria have fibrillae, and gram-negative bacteria have fimbriae and glycocalyx that adhere to specific proteins on the damaged corneal epithelium. After adhering to the epithelium, they release proteases and exotoxins that cause the basement membrane to degrade and to penetrate and lyse the epithelial cells. In the meantime, the bacteria multiplies and invades the stroma. After active ulceration, the inflammatory response regresses, resulting in cicatrization if the infection breached Bowman’s layer.
The different bacteria have characteristic clinical ocular presentations. Gram-positive bacteria tend to produce small distinct abscess lesions. Staphylococcus aureus bacterial keratitis presents with suppuration and yellow-white opaque deep stromal abscesses. Staphylococcus epidermidis keratitis may also have stromal abscesses but not to the severity of Staphylococcus aureus.32 Streptococcus pneumoniae corneal infections appear as central oval stromal ulcers with a progressive edge while another edge is healing. They cause posterior corneal abscesses and anterior chamber hypopyon. Gram-negative bacteria cause diffuse, rapidly spreading necrotic lesions.
Pseudomonas aeruginosa has a characteristic greenish-yellow discharge with severe inflammation and a rapidly progressing ulcer that may be accompanied by stromal melting, infiltrative rings, hypopyon and endothelial plaque. Klebsiella keratitis has whitish-gray pleomorphic suppuration with a diffuse stromal haze. Moraxella causes severe oval stromal ulceration with a necrotic edge, corneal perforation, mild to moderate anterior chamber reaction and hypopyon.33 Neisseria gonorrhea ocular infections present with hyperpurulent conjunctivitis, chemosis and a rapidly progressive stromal infiltration.
Protozoa: Acanthamoeba. This is a common free-living protozoan amoeba that resides in soils and unchlorinated bodies of fresh water. Acanthamoeba can cause sight-threatening keratitis in both non-CL wearers and CL wearers, comprising 5% of lens-related keratitis.34 Acanthamoeba keratitis (AK) affects non-CL wearers who are regularly exposed to dust, soil or contaminated water.35,36 In lens wearers, AK occurs through poor lens hygiene, swimming with CLs, washing lenses in tap water, topping off CL solutions and using ineffective lens solutions.10,37
The adherent surface of soft lenses allows Acanthamoeba access to the cornea. Trophocytes adhere to the increased expression of mannose glycoproteins on the surface of injured corneal epithelium.38 Once attached, they release proteases that are extremely cytolytic to the corneal epithelial cells. They also rapidly encyst themselves into a double wall configuration that is more resistant to destruction. Once past the epithelium, the trophocytes can then breach Bowman’s layer and the stroma, making it difficult for topical amoebicidal medications to penetrate.
Early AK is often misdiagnosed. It begins with a “dirty” looking epithelium and pseudodendritic epitheliopathy characterized by epithelial microcysts and erosions.39 Late AK can have multiple stromal infiltrates, ring infiltrates, scleritis, anterior synechiae, mature cataract and chorioretinitis.39
Parasite: Onchocerca volvulus. According to the World Health Organization, onchocerciasis, or river blindness, affects approximately 17.7 million people, of whom 270,000 are blind and 500,000 have severe visual impairment.40 Onchocerciasis is endemic to West Africa, certain countries in Latin America and Yemen. Simulium black flies that are infected with Onchocerca volvulus release the parasitic larvae when they feed on the blood of humans, livestock and birds. The larvae become adult worms, and the females release approximately 1,000 microfilariae each day over a two-week period.41 The exact pathophysiology of how the microfilariae migrate from the skin and penetrate the conjunctiva is not known; from the conjunctiva, they migrate to the cornea.42 It is not the parasite but its death and the release of its own antigens that initiates the inflammatory response.
Onchocerca volvulus affects the posterior part of the eye as atrophy of the retinal pigmented epithelium. In anterior disease, motile worms can be seen by slit lamp biomicroscopy in the cornea or anterior chamber. When the parasites die, the inflammatory response is initiated causing epithelial punctate keratitis. With continual exposure to the parasite, the inflammatory response results in opacities as it moves posteriorly to the stroma, ultimately resulting in sclerosing keratitis with irreversible blindness.43
Fungi. Since fungi do not inherently penetrate a healthy corneal epithelium, fungal keratitis generally occurs after corneal epithelial trauma from vegetative matter. Fusarium, a filamentous septated fungus, is the most common cause of CL-related fungal keratitis, followed by Aspergillus and Candida.44 The fungus secretes toxins and enzymes, including serine proteases and matrix metalloproteinases, to invade and colonize the cornea. The motile trophozoite can also encyst rapidly into a double-walled configuration, making it highly resistant to destruction. Associated corneal inflammatory reactions from polymorphonuclear leukocytes cause further damage to corneal tissue.45 These infections are hard to eradicate as they penetrate deeper into the cornea where there is less exposure to topical antifungal medications. Clinically, fungal keratitis appears as an ulcer with elevated firm hyphae lines that extend beyond the edge of the central ulcer and feathery stromal lesions. Fungal keratitis represents 1% to 39% of corneal infections in the US.46
Oomycete-Pythium insidiosum (PI). Pythium keratitis is a vision-threatening ocular disease caused by PI, an aquatic oomycete found in tropical and subtropical climates, especially in India and Thailand. Clinically, morphologically and histopathologically, PI keratitis presents very similarly to fungal keratitis. Direct exposure of the cornea to its infectious zoospores from contaminated water or plant material can result in the disease.47 The zoospores encyst on wounded corneal epithelium and secrete glycoproteins that facilitate surface adhesion. The host’s warm body temperature further acts as a stimulus for these zoospores to grow hyphae, which extends on the infected tissue ulcerating the epithelium and penetrates deeper into the cornea.
Clinically, PI keratitis lacks the significant purulence associated with bacterial keratitis. It can present with different patterns of infiltration, the most common having a full thickness stromal infiltrate in the center of the cornea with tentacle-like feathery reticular infiltrates in the subepithelial layer or the anterior stroma.
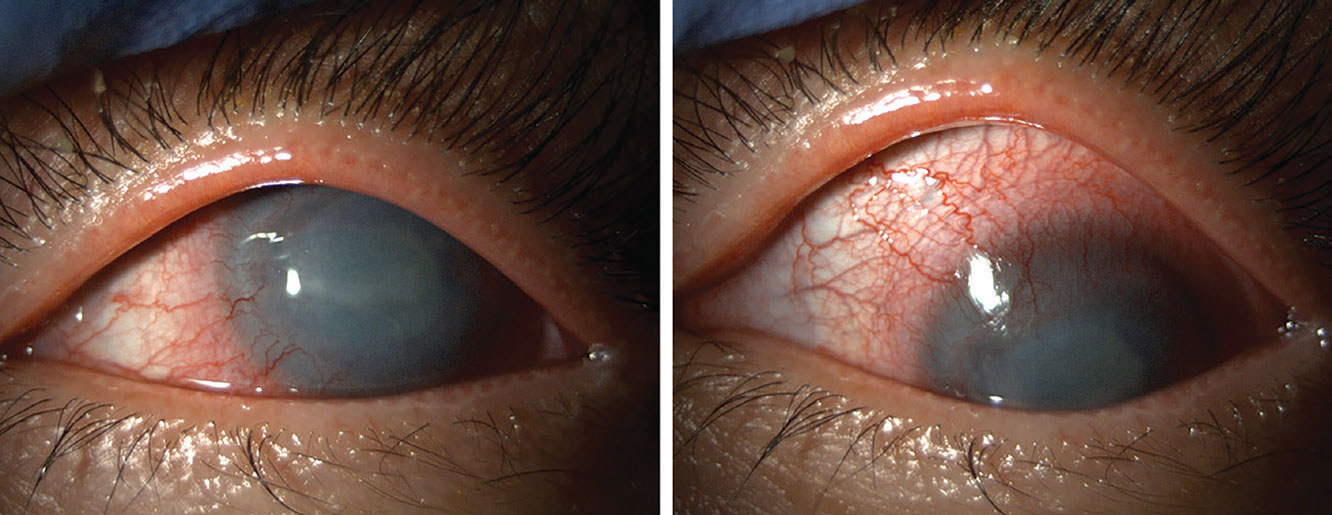 |
AK and conjunctival injection. Photo: Suzanne Sherman, OD. Click image to enlarge. |
Dystrophies
The latest International Committee for Classification of Corneal Dystrophies classification of corneal dystrophies identified dystrophies that (1) affect the epithelium and subepithelium, (2) are epithelial-stromal transforming growth factor beta-induced, (3) are stromal and (4) include endothelial dystrophies.48 Those that affect the epithelium commonly have epithelial defects, which cause associated corneal pain, epiphora and hyperemia. Symptoms range from mild to severe, requiring medical or surgical intervention, and generally tend to recur.
EBMD. Also known as map-dot-fingerprint dystrophy, anterior basement membrane dystrophy and Cogan’s microcystic corneal dystrophy, EBMD is the most common of the corneal dystrophies and one of the most likely causes of RCE. It affects between 2% and 43% of the population, though the exact percentage is not known since many patients are asymptomatic. It generally develops between the ages of 20 and 50 and occurs more in females than males. EBMD is a degenerative condition with, in rare cases, an autosomal dominant mode of inheritance.49 It generally occurs bilaterally but can be unilateral or asymmetrical in presentation.
In EBMD, the basal epithelial cells are dysfunctional, and this results in the formation of an abnormal epithelial basement membrane. There is also an accumulation of fibrillogranular material between the basement membrane and Bowman’s layer and within the epithelium itself.50 The epithelial basement membrane is thicker, multilaminar and protrudes into the epithelium.51
The “map” of map-dot-fingerprint dystrophy is the affected epithelium appearing as large, slightly gray outlines as viewed through the slit lamp. The “dots” are epithelial cells that normally migrate to the corneal surface but get trapped beneath a defective basement membrane that had protruded into the epithelium. Less regularly, the irregular basement membrane forms “fingerprints” of concentric lines in the central cornea. Epithelial cells anterior to the defective basement membrane have difficulty making healthy hemidesmosomes and basement membrane complexes that normally attach to the underlying stroma. This results in RCEs.
Of note, 10% to 33% of EBMD patients have RCEs while 50% of patients with RCEs have EBMD.5,52 Symptoms of EBMD can range from asymptomatic to debilitating and fluctuate with corneal involvement. They include fluctuating vision, glare, distortion, photophobia and foreign body sensation.53
Epithelial recurrent erosion dystrophy (ERED). This includes several distinct dystrophies including Franceschetti corneal dystrophy, Dystrophia Helsinglandica and Dystrophia Smolandiensis. They are rare autosomal dominant conditions characterized by small gray anterior stromal flecks and 0.2mm to 1.5mm diameter whitish-gray, disc-shaped, circular or wreath-like lesions with central clarity in Bowman’s layer and the immediate subjacent anterior stroma.54
Patients with ERED have frequent painful RCEs caused by impaired epithelial adherence. It generally occurs in the first decade of life but decreases in frequency and severity by 30 to 40 years of age.55 The frequency and severity of the erosions may cause severe subepithelial scarring and fibrosis resulting in irregular corneas. Given the rarity and unfamiliarity with ERED, the majority of affected patients are either misdiagnosed or not given a specific diagnosis.
 |
| Click table to enlarge. |
Subepithelial mucinous corneal dystrophy (SMCD). This is a rare autosomal dominant epithelial corneal dystrophy currently identified in one single family. Bilateral subepithelial nodular opacities and corneal haze affect the entire cornea but are more dense centrally. These are accompanied by RCEs. It appears during the first decade of life, and vision may decrease after the fifth decade of life. Clinically, it resembles Grayson-Wilbrandt dystrophy but differs histochemically.56
Meesmann corneal dystrophy (MECD). Also known as juvenile epithelial dystrophy, MECD is a rare autosomal dominant corneal epithelial dystrophy characterized by small, round microcysts diffusely distributed at different levels in the epithelium. The epithelium may be thickened and disorganized. Microcysts develop during childhood, but MECD often remains asymptomatic until after the age of 40 with the occurrence of corneal erosions, photophobia, excessive lacrimation and decreased visual acuity through the irregular cornea.57
Clinical Pearls
Maintaining corneal epithelium health is vital, and it is our job as eyecare professionals to educate patients on how to keep their eyes healthy. When compromised, the epithelium exposes the rest of the eye to a wide variety of insults that can be sight-threatening. Early diagnosis, detailed clinical history, excellent slit lamp biomicroscopy skills and a breadth of treatment options can considerably affect a patient’s vision and quality of life.
Dr. Yeung is a senior optometrist at the UCLA Arthur Ashe Student Health and Wellness Optometry Clinic. She is a diplomate of the American Academy of Optometry Cornea, Contact Lens and Refractive Technologies Section.
Ms. Snyder is a senior biochemistry undergraduate student at UCLA. She currently works as an optician assistant at the UCLA Student Health and Wellness Optometry Clinic. They have no relevant financial interests to disclose.
1. Bergmanson JPG. Anatomy and Physiology of the Cornea and Related Structures. 2019; doi:10.1016/B978-0-7020-7168-3.00003-9. 2. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood) 2001;226(7):653-64. 3. Shields T, Sloane PD. A comparison of eye problems in primary care and ophthalmology practices. Fam Med 1991;23(7):544-6. 4. Suri K, Kosker M, Duman F, et al. Demographic patterns and treatment outcomes of patients with recurrent corneal erosions related to trauma and epithelial and bowman layer disorders. Am J Ophthalmol 2013;156(6):1082-7. 5. Reidy JJ, Paulus MP, Gona S. (2000). Recurrent erosions of the cornea: epidemiology and treatment. Cornea 2000;19(6):767-71. 6. Hykin PG, Foss AE, Pavesio C, et al. The natural history and management of recurrent corneal erosion: a prospective randomised trial. Eye (Lond) 1994;8( Pt 1):35-40. 7. Tripathi RC, Bron AJ. Ultrastructural study of non-traumatic recurrent corneal erosion. Br J Ophthalmol 1972;56(2):73-85. 8. Wilson SE, Medeiros CS, Santhiago MR. Pathophysiology of Corneal Scarring in Persistent Epithelial Defects After PRK and Other Corneal Injuries. J Refract Surg 2018;34(1):59-64. 9. Gurnani B, Kaur K. Bacterial Keratitis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022; https://www.ncbi.nlm.nih.gov/books/NBK574509/. 10. Siddiqui R, Lakhundi S, Khan NA. Status of the effectiveness of contact lens solutions against keratitis-causing pathogens. Cont Lens Anterior Eye 2015;38(1):34-8. 11. Keay L, Edwards K, Naduvilath T, et al. Factors affecting the morbidity of contact lens–related microbial keratitis: A population study. Invest Ophthalmol Vis Sci 2006 Oct;47(10):4302-8. 12. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf 2017;15(3):334-65. 13. Farris RL, Stuchell RN, Mandel ID. Tear osmolarity variation in the dry eye. Trans Am Ophthalmol Soc 1986;84:250-68. 14. Chen Z, Tong L, Li Z, et al. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49(2):539-49. 15. Lee SY, Petznick A, Tong L. Associations of systemic diseases, smoking and contact lens wear with severity of dry eye. Ophthalmic Physiol Opt. 2012;32(6):518-26. 16. Thygeson P. Superficial punctate keratitis. J Am Med Assoc. 1950;144(18):1544-9. 17. Quere MA, Delplace MP, Rossazza C, et al. Fréquence et étiopathogénie de la kératite de Thygeson. Bull Soc Ophtalmol Fr. 1973;73(4):629-31. 18. Singh K, Axelrod S, Bielory L. The epidemiology of ocular and nasal allergy in the United States, 1988-1994. J Allergy Clin Immunol. 2010;126(4):778-83. 19. Yokoi K, Yokoi N, Kinoshita S. Impairment of ocular surface epithelium barrier function in patients with atopic dermatitis. Br J Ophthalmol. 1998;82(7):797-800. 20. Wilson SE. Laser in situ keratomileusis–induced (presumed) neurotrophic epitheliopathy. Ophthalmology. 2001;108(6):1082-7. 21. Bonini S, Lambiase A, Rama P, et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347-51. 22. Gilbard JP, Rossi SR. Tear film and ocular surface changes in a rabbit model of neurotrophic keratitis. Ophthalmology. 1990;97(3):308-12. 23. Alper MG. The anesthetic eye: an investigation of changes in the anterior ocular segment of the monkey caused by interrupting the trigeminal nerve at various levels along its course. Trans Am Ophthalmol Soc. 1975;73:323-65. 24. Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74(24):11464-71. 25. Chodosh J, Miller D, Stroop WG, et al. Adenovirus epithelial keratitis. Cornea. 1995;14(2):167-74. 26. Lee CS, Lee AY, Akileswaran L, et al. Determinants of outcomes of adenoviral keratoconjunctivitis. Ophthalmology. 2018;125:1344-53. 27. Cope JR, Konne NM, Jacobs DS, et al. Corneal Infections Associated with Sleeping in Contact Lenses - Six Cases, United States, 2016-2018. MMWR. Morbidity and mortality weekly report, 2018;67(32):877-81. 28. Stapleton F, Naduvilath T, Keay L, et al. Risk factors and causative organisms in microbial keratitis in daily disposable contact lens wear. PLoS One. 2017;12(8):e0181343. 29. Ranjini CY, Waddepally VV. Microbial profile of corneal ulcers in a tertiary care hospital in South India. J Ophthalmic Vis Res. 2016;11(4):363-7. 30. Vallas V, Stapleton F, Wilcox M. Bacterial invasion of corneal epithelial cells. Australian and New Zealand Journal of Ophthalmology. 1999;27(3-4):228-30. 31. Dini LA, Cockinos C, Frean JA, et al. Unusual case of Acanthamoeba polyphaga and Pseudomonas aeruginosa keratitis in a contact lens wearer from Gauteng, South Africa. J Clin Microbiol. 2000;38(2):826-9. 32. Liesegang TJ. Corneal complications from herpes zoster ophthalmicus. Ophthalmology. 1985;92(3):316-24. 33. Das S, Constantinou M, Daniell M, et al. Moraxella keratitis: predisposing factors and clinical review of 95 cases. Br J Ophthalmol. 2006;90(10):1236-8. 34. Stern GA, Lubniewski A, Allen C. The interaction between Pseudomonas aeruginosa and the corneal epithelium: An electron microscopic study. Arch Ophthalmol. 1985;103(8):1221-5. 35. Manikandan P, Bhaskar M, Revathy R, et al. Acanthamoeba keratitis–A six year epidemiological review from a tertiary care eye hospital in South India. Indian Journal of Medical Microbiology. 2004;22(4):226-30. 36. Garg P, Kalra P, Joseph J. Non-contact lens related Acanthamoeba keratitis. Indian J Ophthalmol. 2017;65(11):1079-86. 37. Silvany RE, Dougherty JM, McCulley JP. Effect of contact lens preservatives on Acanthamoeba. Ophthalmology. 1991;98(6):854-7. 38. Garate M, Cao Z, Bateman E, et al. Cloning and characterization of a novel mannose-binding protein of Acanthamoeba. J Biol Chem. 2004;279(28):29849-56. 39. Szentmáry N, Daas L, Shi L, et al. Acanthamoeba keratitis - Clinical signs, differential diagnosis and treatment. J Curr Ophthalmol. 2018;31(1):16-23. 40. Hoerauf A, Büttner DW, Adjei O, et al. Onchocerciasis. BMJ. 2003;326(7382):207-10. 41. Schulz-Key H. Observations on the reproductive biology of Onchocerca volvulus. Acta Leiden. 1990;59(1-2):27-44. 42. Duke BO, Anderson J. A comparison of the lesions produced in the cornea of the rabbit eye by microfilariae of the forest and Sudan-savanna strains of Onchocerca volvulus from Cameroon. I. The clinical picture. Z Tropenmed Parasitol. 1972;23(4):354-68. 43. Hall LR, Pearlman E. Pathogenesis of onchocercal keratitis (River blindness). Clin Microbiol Rev. 1999;12(3):445-53. 44. Acharya Y, Acharya B, Karki P. Fungal keratitis: study of increasing trend and common determinants. Nepal J Epidemiol. 2017;7(2):685-93. 45. Gopinathan U, Ramakrishna T, Willcox M, et al. Enzymatic, clinical and histologic evaluation of corneal tissues in experimental fungal keratitis in rabbits. Exp Eye Res. 2001;72(4):433-42. 46. Gower EW, Keay LJ, Oechsler RA, et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117(12):2263-7. 47. Mendoza L, Hernandez F, Ajello L. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. Journal Clin Microbiol. 1993;31(11):2967-73. 48. Weiss JS, Møller HU, Aldave AJ, et al. IC3D classification of corneal dystrophies—edition 2. Cornea. 2015;34(2):117-59. 49. Boutboul S, Black GC, Moore JE, et al. A subset of patients with epithelial basement membrane corneal dystrophy have mutations in TGFBI/BIGH3. Hum Mutat. 2006;27(6):553-7. 50. Francois J, Hanssens M, Teuchy H, et al. Ultrastructural findings in corneal macular dystrophy (Groenouw II type). Ophthalmic Research. 1975;7(2):80-98. 51. Wood TO, Griffith ME. Corneal epithelial basement membrane dystrophy. Trans Am Ophthalmol Soc. 1987;85:281-92. 52. Bron AJ, Brown NAP. (1976). Recurrent corneal erosion. In New Developments in Ophthalmology Nijmegen 16–18 October 1975 (pp. 27-38). Springer, Dordrecht. 53. Miller DD, Hasan SA, Simmons NL, et al. Recurrent corneal erosion: a comprehensive review. Clin Ophthalmol. 2019;13:325-35. 54. Oliver VF, van Bysterveldt KA, Cadzow M, et al. A COL17A1 Splice-Altering Mutation Is Prevalent in Inherited Recurrent Corneal Erosions. Ophthalmology. 2016;123(4):709-22. 55. Vahedi F, Chung DD, Gee KM, et al. Epithelial Recurrent Erosion Dystrophy Secondary to COL17A1 c.3156C>T Mutation in a Non-white Family. Cornea. 2018;37(7):909-11. 56. Feder RS, Jay M, Yue BY, et al. Subepithelial mucinous corneal dystrophy: clinical and pathological correlations. Arch Ophthalmol.1993;111(8):1106-14. 57. Cogan DG, Donaldson DD, Kuwabara T, et al. Microcystic dystrophy of the corneal epithelium. Trans Am Ophthalmol Soc. 1964;62:213-25. |


