With ongoing advancements, we have seen growing interest in the area of smart contact lenses and drug-eluting lenses. While there’s still much to discover, the potential implications of these technologies paint an exciting future for eye care.
“Many years and decades of discussion are now becoming actuality with the new Acuvue Theravision lens with ketotifen that was recently released,” by Johnson & Johnson says Rebecca Rojas, OD, of Columbia University, Harkness Eye Institute. She notes that this “opens the door for other drug-eluting lenses and smart lens trials to follow.”
This article will delve into current research and development efforts, as well as associated challenges and hopes for the future of smart contact lenses and drug-eluting technology.
 |
 |
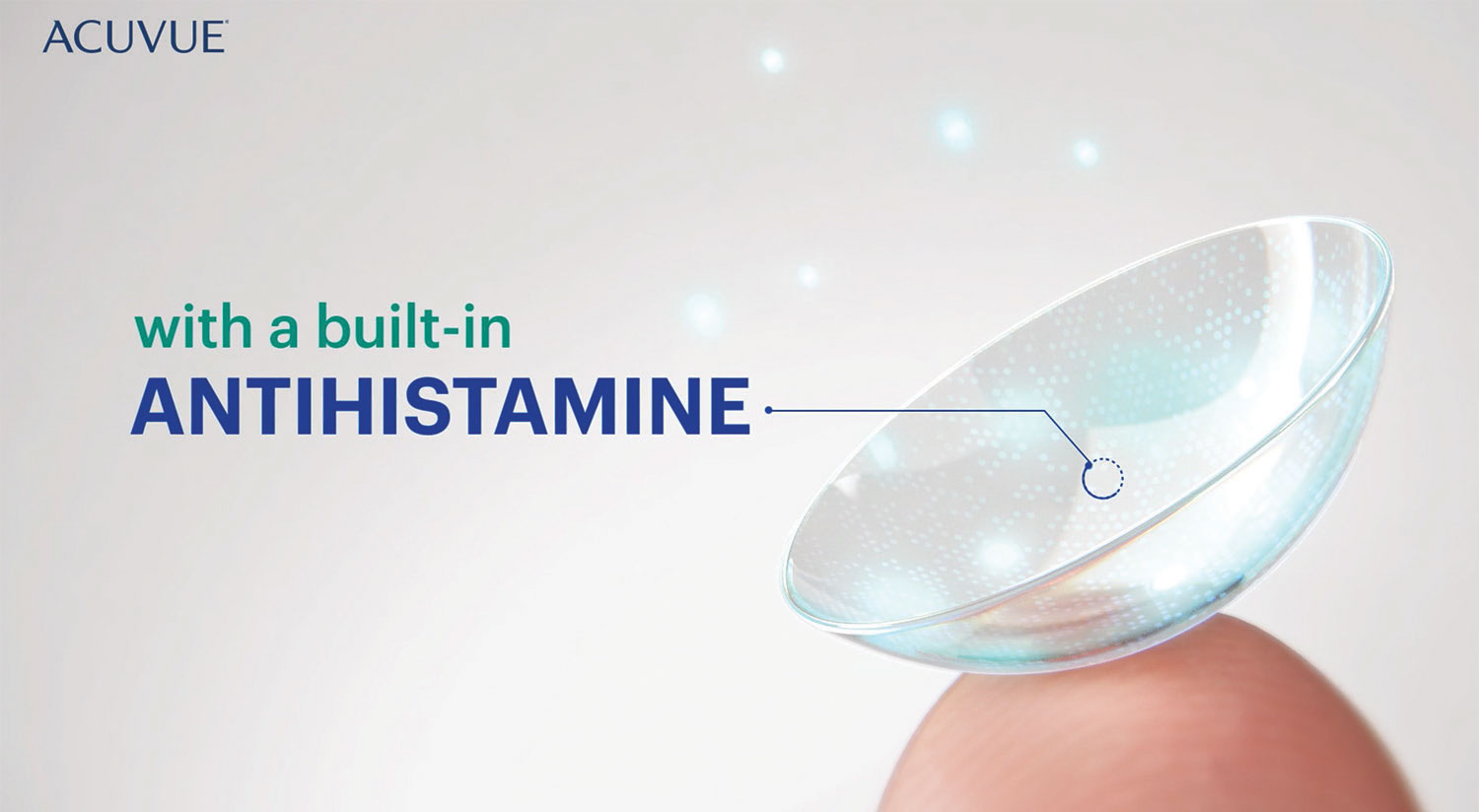
| The mainstays of drug delivery—bottles and droppers—can make instillation challenging for patients. So far, there’s one drug-eluting contact lens available, Johnson & Johnson’s Acuvue Theravision, and others are anticipated. Photo: Melissa Barnett, OD. Click images to enlarge. |
Where We Stand
Both drug-eluting lenses and smart contacts have seen increasing momentum in recent years, and efforts to capture the potential of these technologies are in full force.
Drug-eluting lenses. The concept of contact lenses as ocular drug-delivery systems is not new; however, with ongoing advances, it is now becoming a reality. There are a number of ways to develop therapeutic contact lenses, according to Melissa Barnett, OD, of the University of California, Davis Eye Center. These include the soaking method, molecular imprinting, colloidal nanoparticle-laden lenses and using vitamin E.
“Drug-eluting contact lenses are unique, given each pharmaceutical formulation presents different challenges with regard to demonstrating safety and efficacy for the respective indication,” notes Jerry Legerton, OD, co-founder and chief clinical and regulatory officer of Innovega. “In some cases, the pharmaceutical must be reformulated and diluted for constant presence during the continuous elution.
“Other challenges include management of shelf life of the drug-loaded contact lenses, and their respective efficacy gradients may be impacted by aging over time. This in turn impacts the labeled shelf life,” he explains. “There are multiple strategies for making drug-eluting contact lenses, including, but not limited to, surface printing, nanoparticles, polymer structure to accommodate molecule size and shape and microfluidics.”
In March 2022, Johnson & Johnson’s Acuvue Theravision (etafilcon A drug-eluting contact lens with ketotifen) was the first drug-eluting contact lens to receive FDA approval. Each daily disposable lens contains 19mcg of ketotifen and is indicated for the prevention of ocular itching due to allergic conjunctivitis. Phase III clinical trial findings showed a meaningful reduction in itchy eyes with allergies as quickly as three minutes after lens insertion and lasting up to 12 hours.1
John Gelles, OD, director of the Specialty Contact Lens Division at the Cornea and Laser Eye Institute (CLEI) and the CLEI Center for Keratoconus in New Jersey, says that he’s heard this therapeutic contact lens has a “good effect for individuals who have mild allergies.” While this approach so far appears to be less effective for patients with more moderate to severe allergies, he notes that this is a “big win for those with mild cases.”
This approval—and others like it in the future—helps address a significant issue associated with eye drops: patient compliance. “This is a huge driver in these efforts,” says Dr. Gelles, who is also a clinical assistant professor at Rutgers New Jersey Medical School, Department of Ophthalmology and Visual Science. “No one who needs correction is going to go without correction throughout the day. However, that same person will skip their drops because they’re inconvenient. So, if we can put their medication into lenses that they absolutely need, this can be very effective.”
Another area where drug-eluting contact lenses are being explored is glaucoma. The SIGHT (Sustained Innovative Glaucoma and ocular Hypertension Treatment) clinical program aims to treat mild to moderate glaucoma and ocular hypertension. The phase IIa SIGHT-1 trial evaluated LLT-BMT1 (MediPrint Ophthalmics), a drug-eluting lens that uses bimatoprost.
“The study demonstrated strong safety signals with 100% tolerability and no significant adverse events,” according to Dr. Barnett, who discussed drug-eluting lenses in a previous Review of Optometry article. “The researchers also found that the incidence of hyperemia among study participants was lower than what is observed for bimatoprost drops—a standard of care approach for this condition.”2
Based on these findings, in November 2022, the company announced the initiation of SIGHT-2, a dose-finding phase IIb study. There are also plans to continue exploring the potential of LLT-BMT1 in phase III clinical trials.
Contact lenses could also prove to be an effective vehicle for latanoprost—an FDA-approved agent for the treatment of elevated intraocular pressure (IOP) in glaucoma patients. Preclinical data has shown that continued delivery of latanoprost via contact lenses is at least as effective as daily latanoprost ophthalmic solution, according to Dr. Barnett.2 A phase I study is currently underway, aiming to explore the safety, tolerability, comfort and feasibility of lowering IOP in glaucoma patients using latanoprost-eluting contact lenses (NCT04500574).
There are a number of other avenues currently under investigation, including lenses that deliver anti-inflammatory, antibiotic and pain-reducing drugs. This could be beneficial for patients undergoing ocular surgery as well as those with corneal abrasions. Another area where drug-eluting lenses could have a significant impact is in the treatment of ocular inflammation. A dexamethasone-releasing contact lens has shown promise in animal models. “While more research is needed, dexamethasone-eluting lenses could prove to be an effective treatment for ocular inflammation and a promising drug-delivery system,” says Dr. Barnett.2
“Drug-releasing contact lenses may be beneficial for certain patients who have difficulty with compliance when drops are required multiple times a day,” says Dr. Rojas. “For others, instillation of eye drops also poses a challenge.
“For age-related conditions that affect the same population, insertion and removal of contact lenses may still be just as difficult due to dexterity issues or poor vision,” she adds. “Taking those considerations into account, it is evolving to where lens use may not be needed daily but for lesser wear time with extended-release capabilities up to several days after one day of wear.”
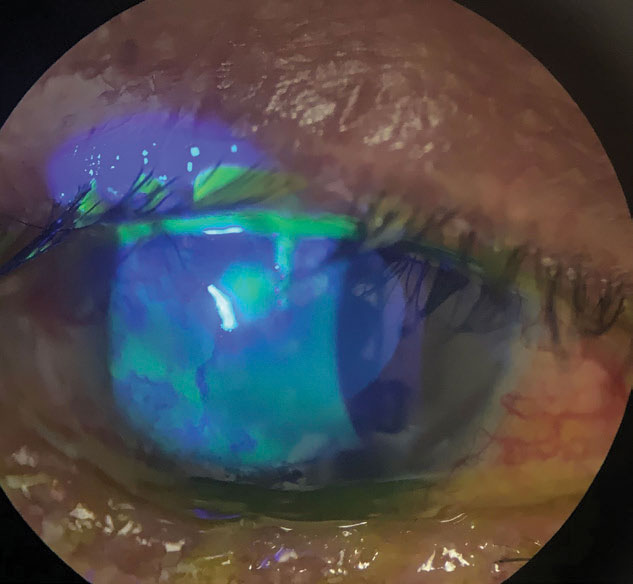 |
Antibiotics in the bowl of a scleral could help heal persistent epithelial defects and neurotrophic keratitis, as seen in this eye. Photo: Melissa Barnett, OD. Click image to enlarge. |
Smart contact lenses. The field of smart contact lenses is an exciting one that could have an array of implications for eye care; however, we are still in the early stages of innovation. “Smart contact lens development is very difficult and has consumed as much as $1 billion in research and development to-date,” notes Dr. Legerton.
“Two of the big four contact lens companies invested heavily for the better part of a decade in smart contact lens development and solved many of the important pieces required for placing electronics and micro-electro-mechanical-systems in contact lens materials,” he says. He adds that the lenses advanced through phase II clinical investigations and stopped short of the phase III pivotal investigations needed for market clearance or approval.
“Generally speaking, they established efficacy for the indications they were pursuing in accommodating contact lenses and sensing of constituents in the tear film that are indicators of ocular or systemic health status,” says Dr. Legerton. “Why did they not take the next steps to market clearance or approval?”
While the reasons for this are likely multifaceted, a necessary component of any commercialized product is that it must be both useful and usable. “When we describe a smart contact lens, we place a great emphasis on its usefulness or purpose. We envision what it does, its purpose and benefit to humankind and the problem it solves,” says Dr. Legerton. “At the same time, the final product must be usable.”
This holds true for smart contact lenses, as well. Just as these innovations need to address a problem and/or enhance quality of life, they must also not disturb vision. They need to be easy to handle, apply and remove. Additionally, the care product regimen must be convenient and usual, notes Dr. Legerton, and it must have an acceptable cost-to-benefit ratio.
Ongoing innovation is paving the way for the development of useful—and usable—smart contact lenses, and a variety of new directions are currently being explored. For instance, researchers have developed a smart contact lens that continually monitors an individual’s blood sugar—a device that could have significant clinical implications if successfully brought to market.
“Smart contact lenses for continuous glucose monitoring have great potential for huge clinical impact. To date, their development has been limited by challenges in accurate detection of glucose without hysteresis for tear glucose monitoring to track the blood glucose levels,” the researchers note in a recent Advanced Materials paper.3
Long-term continuous glucose monitoring was conducted in preclinical models using bimetallic nanocatalysts immobilized in nanoporous hydrogels in smart contact lenses, according to one study. “After redox reaction of glucose oxidase, the nanocatalysts facilitate rapid decomposition of hydrogen peroxide and nanoparticle-mediated charge transfer with drastically improved diffusion via rapid swelling of nanoporous hydrogels.”
Another team of investigators developed a smart contact lens that can monitor and control IOP by combining an IOP sensor and a flexible drug-delivery system. Recently published findings showed that their theranostic smart contact lens enabled both IOP measurements in real-time and the appropriate amount of drug release to match the degree of IOP among rabbits with glaucoma.4
The concept of not only measuring but also controlling IOP via one system is exciting, Dr. Gelles notes. However, it also raises important questions, particularly in regard to extended wear. “We know the risks that are associated with extended contact lens wear, and we are going to have to find ways to mitigate those risks.”
Innovega’s iOptik smart contact lens is another example of where the field is headed. “The iOptik lens represents what we call eyeborne optics that enable the eye to see the real world and to view a near eye display without any other optics between the eye and the display,” explains Dr. Legerton.
This technology is the first to reduce the bulk and weight in the display eyewear, according to Dr. Legerton. He notes that “all VR headsets and most AR glasses employ optics between the display and the eye. The iOptik lens allows for removal of those optics and direct viewing of the display while still seeing the real world with corrected vision.”
Previously released findings from a phase II clinical trial “demonstrated positive results with normally sighted subjects fit with iOptik smart contact lenses when tested both with and without the display eyewear,” according to the company.5
Another study of the eMacula system—which pairs the iOptik smart contact lenses with display eyewear—showed its potential for helping partially sighted individuals with their daily tasks, including reading, smartphone use and distance.6
Results showed that visual acuity was improved in each eye tested with the device. “Study participants also rated the comfort of the smart contact lenses at an average of 7.1 on a scale of one (poor) to 10 (excellent),” according to findings released by the company. “Three-quarters of subjects felt the device would likely improve performance on tasks of daily living and increase their independence.”
These are just a handful of the various areas currently being explored and developed; the potential implications of smart contact lenses have just started to be fully realized. However, Dr. Rojas believes they could be particularly impactful for certain patients.
“It’s especially exciting for low vision patients to implement the most up-to-date technology to not only improve their vision but also their quality of life,” she says. She reiterates the benefits of other smart lenses, including “the ability to self-monitor and track IOP or glucose and/or provide extended-release drug-delivery options, which are beneficial in diagnosis and treatment plans.”
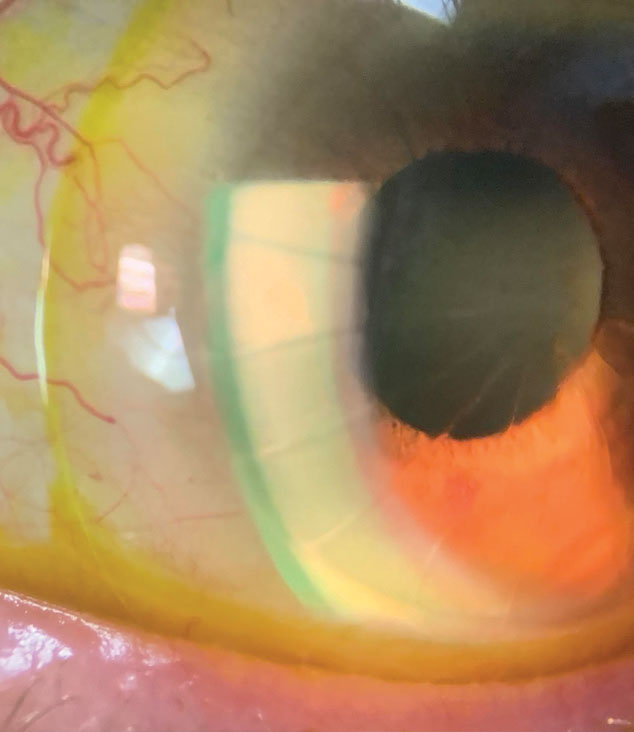 |
A scleral with sodium fluorescein on an eye with radial keratectomy, lipid keratitis and neovascularization. Photo: Melissa Barnett, OD. Click image to enlarge. |
Other Challenges & Considerations
While the innovation behind these contact lenses is exciting and holds promise for the future of eye care, the developmental process isn’t without its hurdles, some that will certainly prove more challenging to navigate than others.
An example is Mojo Vision’s recent pivot away from smart contact lenses. The company attempted to develop a scleral contact lens that included electronic components for projecting display light through the eye to the retina, explains Dr. Legerton. “The scleral contact lens form factor was, of necessity to house the electronics, approximately 2mm thick, or six-times the average scleral contact lens design.”
This underscores the importance of usefulness and usability. “While the vision and mission of Mojo Vision was exciting, the practical element of using an ultra-thick scleral contact lens as the form factor was a showstopper from the start with regard to the otherwise useful technology to be usable,” says Dr. Legerton. He notes that the hope for success from the start depended on miniaturization.
“It is noteworthy that miniaturization is a major factor in the failure of smart glasses for extended reality to be accepted,” he adds. “The headsets and glasses are simply too bulky, too heavy and consume too much power. The industry calls this ‘SWAP,’ or size, weight and power.”
Does the display contact lens still have potential? As a self-described futurist, Dr. Legerton says, yes. “That said, futurists see mountain tops and have more difficulty estimating the length of the valleys between the mountain ranges,” he notes. He also emphasizes that “electronics of all types have the potential to be components in a contact lens.”
“The electronics industry has enjoyed miniaturization at an accelerated pace over the last six decades,” Dr. Legerton says. “Mojo Vision made a pivot to exit the contact lens development and become a leader in the miniaturization that is required.”
As hurdles are overcome and advances continue, optometrists will be on the frontlines of integrating new lenses, such as Johnson & Johnson’s Theravision, into clinical practice. This comes with its own set of challenges and considerations, including issues of non-compliance, poor lens hygiene and overwear, according to Dr. Rojas.
“These can lead to further complications of irritation, inflammation, infections or risks to eye health,” she notes. “Just like any medical device placed on any organ of the body, there need to be proper follow-ups to maintain the integrity and health of the eye and prevent any potential complications.”
Optometrists, as they always do, will play a key role in patient education and support. “It is important for both the patient and the doctor to make sure patients are properly informed of the risks associated with improper lens care and wear,” Dr. Rojas says.
“Any type of contact lens can pose a risk to eye health if not properly cared for or not fitted properly,” she emphasizes. “Just like any other medical device, we need to make sure the patient’s health is the priority and whatever device used has benefits that outweigh the risk.”
On the Horizon
What comes next? With ongoing advances and a growing interest in smart contact lenses, the path forward will certainly including multiple directions. And while it likely will be a long road with many twists and turns along the way, smart contact lenses could one day have a significant impact on eye care.
For Dr. Legerton, his personal vision and mission is to “invent and develop technologies that enhance the health, wellness and quality of life for humankind while respecting the value of optometry in the delivery of the technologies.”
While Dr. Rojas isn’t sure smart contact lenses will become mainstream any time soon, as safety and affordability are still important factors to consider, she can see the benefit and initial growth for a specific patient base. “It is exciting to see how far technology has come and the potential it offers to improve patients’ lives,” she says.
1. Pall B, Gomes P, Yi F, et al. Management of Ocular Allergy Itch With an Antihistamine-Releasing Contact Lens. Cornea. 2019;38(6):713-7. 2. Barnett M. The Coming Rise of Drug-delivery Contact Lenses. Review of Optometry. 2021; https://www.reviewofoptometry.com/article/the-coming-rise-of-drugdelivery-contact-lenses. 3. Kim SK, Lee GH, Jeon C, et al. Bimetallic Nanocatalysts Immobilized in Nanoporous Hydrogels for Long-Term Robust Continuous Glucose Monitoring of Smart Contact Lens. Advanced Materials. 2022; https://doi.org/10.1002/adma.202110536. 4. Kim TY, Mok JW, Hong SH, et al. Wireless theranostic smart contact lens for monitoring and control of intraocular pressure in glaucoma. Nature Communications. 2022; https://doi.org/10.1038/s41467-022-34597-8. 5. Positive Visual Performance Reported with Innovega’s iOptik Contact Lens. https://www.prnewswire.com/news-releases/positive-visual-performance-reported-with-innovegas-ioptik-contact-lens-301421115.html. 6. Innovega’s eMacula System Shows Potential to Increase Independence for Visually Impaired. https://www.prnewswire.com/news-releases/innovegas-emacula-system-shows-potential-to-increase-independence-for-visually-impaired-301286652.html. |


