Dry eye disease (DED) affects nearly a quarter of patients seeking eye care in the United States and, for many, commercially available lubricants provide adequate symptom relief. However, artificial tears only augment the lubrication and mechanical clearance provided by natural tears, and they require frequent use with decreased efficacy over time.1,2 In addition, these over-the-counter treatments include preservatives that can cause irritation and fail to supply the ocular surface with the nutrients that support epithelial growth and differentiation.3 They lack the composition—water, salts, hydrocarbons, proteins and lipids—of natural tears.4
Ultimately, these traditional therapies often fail for the most severe sufferers.5 Ocular surface injury is the hallmark of severe DED, and treatments must target surface improvement.5 Today, clinicians can consider prescribing serum tears to meet the needs of patients who fail to improve with other dry eye treatments. Blood serum shares much of the biologic composition of natural tears and can provide essential epithelial support and antimicrobial activity.1 When natural tears are depleted or imbalanced, serum tears—whether autologous, allogeneic or platelet rich plasma (PRP)—play an important role in restoring ocular surface health.
The First Applications of Serum TearsHematopoietic therapy for severe dry eye was first introduced in 1975 for severe ocular burn and Stevens-Johnson Syndrome patients via continuous ocular perfusion of autologous and homologous serum and plasma.1 Later, in 1984, other researchers used serum tears to treat keratitis sicca patients whose signs and symptoms of dry eye persisted with the use of commercial artificial tears.2 All 15 patients enrolled in the study showed improvement in objective and symptomatic findings with serum tear treatment.2 The serum protein was hypothesize to provide the nutrients and bacteriostatic agents required for healing.2
|
A Look Under the Hood
Corneal and conjunctival epithelial cells are imperative for wound healing with their proliferative, migratory and differentiating abilities.1,6,7 The aqueous component of the tear film enables and supports these functions through its mechanical properties and nourishment.1 The antimicrobial role of the tear film is triggered by the inflammation of the ocular surface, as proteins and complement factors are released into the aqueous to participate in microbe destruction via macrophages and lymphocytes.1
When the tear film is deficient, the impaired support system compromises the integrity of the ocular surface. In particular, growth factors known to reduce inflammation are diminished in dry eye patients, and their imbalance may be responsible for dry eye pathogenesis.3,8
Restoring Balance
For moderate to severe DED patients, the chemicals present in serum tears play a significant role in restoring the ocular surface.2 Serum and natural tears share vital components, albeit at differing concentrations, including: epidermal growth factor (EGF), vitamin A, fibronectin and transforming growth factor-β (TGF-β).1
EGF accelerates the proliferation of the corneal epithelium, vitamin A prevents the epithelium from undergoing squamous metaplasia and fibronectin influences cell adhesion and migration during the healing process.1,9 Both fibronectin and vitamin A aid in the integrity of the ocular surface, and the tissue becomes compromised when the tear film is deficient.8
TGF-β is a particularly tricky component to balance in serum tears, considering it suppresses autoimmune reactions at normal tear levels but can cause an inflammatory response and suppress ocular surface wound healing at higher levels.4,10 Because of this, serum tears are diluted to produce a substance analogous to natural tears in its TGF-β concentration.4 Several variations of serum tears exist, most notable being autologous, allogeneic and PRP.
How They’re MadeThe production and storage of serum tears types are relatively alike. Potential donors undergo a rigorous screening process for infectious diseases, such as hepatitis, syphilis and HIV, as well as for circulating drugs and inflammatory mediators.1,11 Children, pregnant women and patients with comorbidities, such as cardiac or pulmonary disease, are also excluded as donors.4 For acceptable donors, whole blood is collected, allowed to clot and then centrifuged.1 Serum is harvested and diluted with sterile saline.1,11 Although post-dilution concentrations vary, 20% is most widely used.1,4 The diluted product is bottled into single-use, preservative-free vials, sealed and stored.1 Unused tears are stored at -20°C for up to three months.1,4 Opened bottles may be kept at 4°C for up to 24 hours.1,4 Serum tears are not regulated by the FDA, as they are a blood product rather than a pharmaceutical.6 Although some state regulations exist, there is no established protocol for the production or use of serum tears.6
|
Autologous serum tears (AST). These are produced from a patient’s own blood sample. A pronounced benefit of this type is the lack of antigenicity.1 AST may be used alone or in conjunction with more traditional therapies, such as commercial topical therapeutics, bandage or scleral contact lenses and punctal plugs.1 Their lack of preservatives makes them a merited alternative to other available topical treatments.
Allogeneic serum tears. These are produced from another patient’s blood and are an equally efficacious alternative for patients not suited for AST, such as those who cannot safely partake in frequent blood sampling.7,10,11 Allogenic tears may also be a good option for patients with decreased EGF concentration in their autologous serum, a common finding in patients with systemic factors influencing dry eye.7,10 An additional benefit of allogeneic serum is the ability to produce it in larger quantities, increasing cost-effectiveness.11 Potential allogeneic donors are of group AB, the universal plasma donor blood type, without A/B antibodies.11
Platelet rich plasma. This type of hematopoietic therapy has 1.5 times higher concentration of platelets than autologous or allogenic serum tears.12,13 Because the elimination of platelets in serum preparation significantly lowers growth factor levels, PRP may hold a therapeutic advantage over the other two types of serum tears.2 To produce PRP, collected whole blood is centrifuged along with an anticoagulant, leaving the plasma and buffy coat portion of blood to be collected, diluted and stored.12,13 Platelet adhesion to damaged tissue releases cytokines and growth factors that expedite healing.12 Research suggests PRP can further enhance the restoration of the ocular surface through more potent proliferative and anti-inflammatory effects than AST and allogeneic serum tears.2
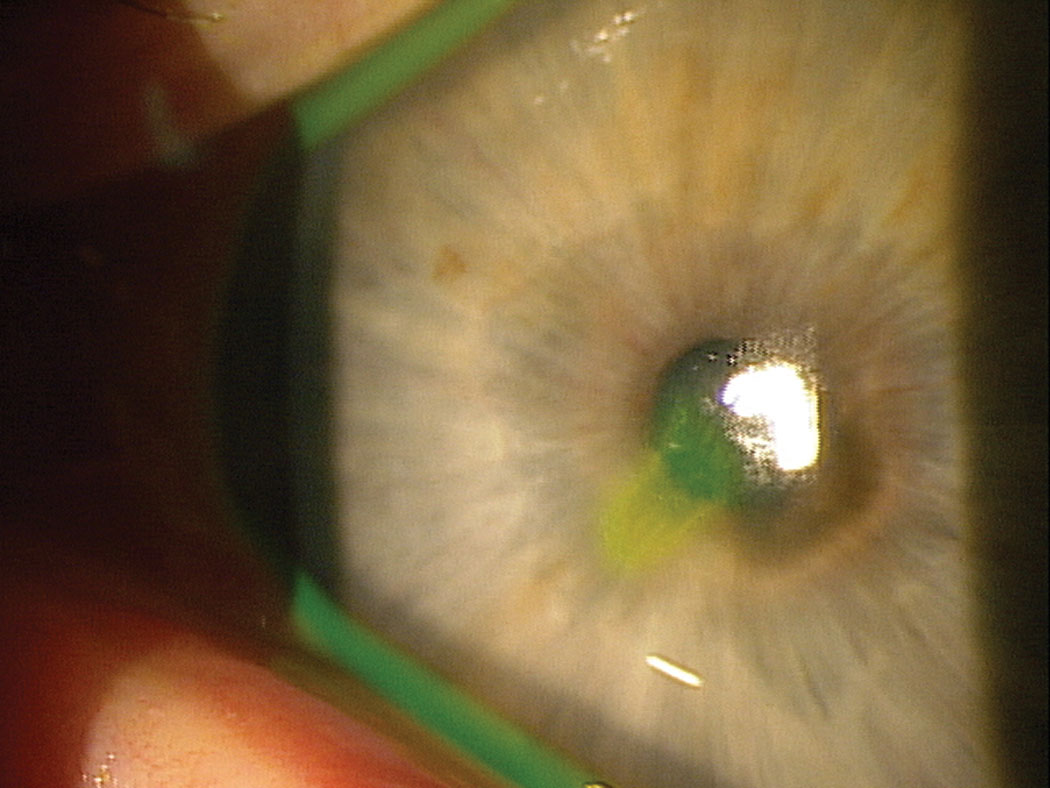 |
| This patient with a non-healing abrasion may benefit from the epithelial support and antimicrobial activity provided by serum tears, and PRP in particular. Click image to enlarge. |
Who and Why to Treat
The 2018 American Academy of Ophthalmology’s Dry Eye Syndrome Preferred Practice Patterns recommends treatment with serum as third-line therapy in the management of dry eye.7 Before initiating serum tears, clinicians should first try environmental and dietary modifications, lid hygiene and both high- and low-viscosity lubricants.7 Second-line therapies to consider before ASTs are preservative-free artificial tears, punctal plugs, moisture chambers, topical corticosteroids, cyclosporine A or LFA-1 antagonists.7
However, some experts favor serum eye drops as a second-line therapy, citing their potential advantages over traditional therapies.14 Although the indications remain limited, serum tears are becoming a more common treatment option, particularly for these conditions:15
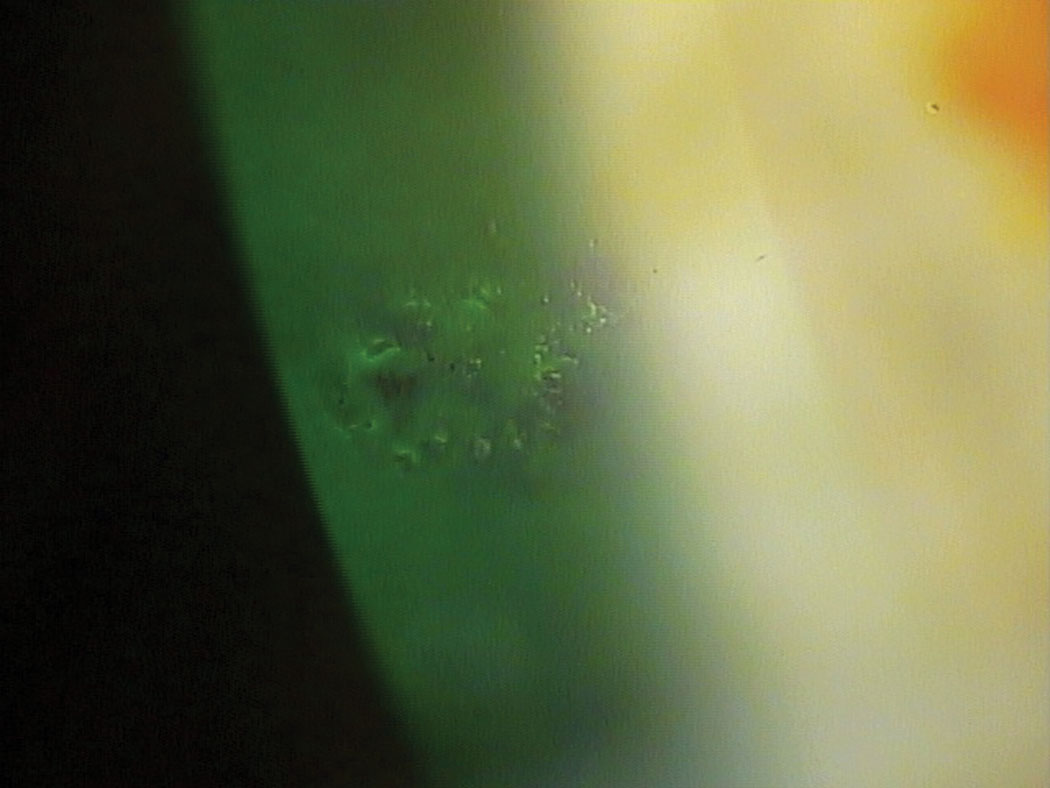 |
| Serum tears can be an important therapeutic option for patients with recurrent corneal erosions. Click image to enlarge. |
Dry eye. Serum tear research is sharply focused on treating severe DED. Succinct prescribing trends have not emerged for reasons that range from discrepancies in disease assessment to variabilities in serum concentration or treatment duration. Recommendations for frequency of application differ as well, varying between three to eight applications per day depending on disease severity.1,4 Despite this, serum tear research has demonstrated therapeutic benefits across the severe DED spectrum.
Patients who are tear deficient due to Sjögren’s syndrome demonstrate improved vital staining with serum tear application.9 In addition, serum tears show a superior efficacy over preservative-free artificial tears through improved tear film and subjective comfort.8,16 Research shows AST concentrations as high as 50% are safe and effective at improving symptoms and Schirmer’s scores.3 Serum drops improve the conjunctival surfaces of dry eye patients as well.14
However, critics of serum tear research cite low evidence certainty and bias risk.4 Added to that, hyperosmolarity is seldom, if ever, measured.4 Because increased osmolarity and surface inflammation is central to the pathogenic mechanism of DED, its absence in study design methods is glaring.7,17
Persistent epithelial defects (PEDs). These also respond well to serum tear treatment. PEDs often occur secondary to rheumatoid arthritis, neurotrophic keratopathy, keratoconjunctivitis sicca and other chronic inflammatory conditions.1,12 One study shows that 63% of PEDs refractory to more than two weeks of treatment with lubricants and bandage contact lenses were completely healed after four weeks of serum tear treatment.1 In addition, 90% of PEDs displayed a reduction in defect size after treatment with serum tears.6
Another study found decreased recurrence of PEDs when serum tears were used four times a day for two weeks following removal of a bandage contact lens.18 PRP was found to result in increased rate of epithelial healing compared with autologous serum in PEDs secondary to infectious keratitis.13
Recurrent corneal erosions (RCEs). Research shows RCEs exhibited decreased recurrence with six months of PRP treatment.13 One study comparing conventional RCE treatment alone (bandage contact lens and preservative-free artificial tears) with the addition of PRP found that 80% of conventional treatment patients and just 26% of PRP patients experienced a major recurrence.13 Minor recurrences were experienced by 100% of conventional treatment patients but only 37% of PRP patients.13
Superior limbic keratitis. This is another condition responsive to serum tear treatment. One study found that 82% of patients reported improvement in discomfort and 100% improvement in epitheliopathy after using 20% serum tears ten times per day for one month compared with previous therapies, such as frequent artificial tears, topical corticosteroids and topical vitamin A.1
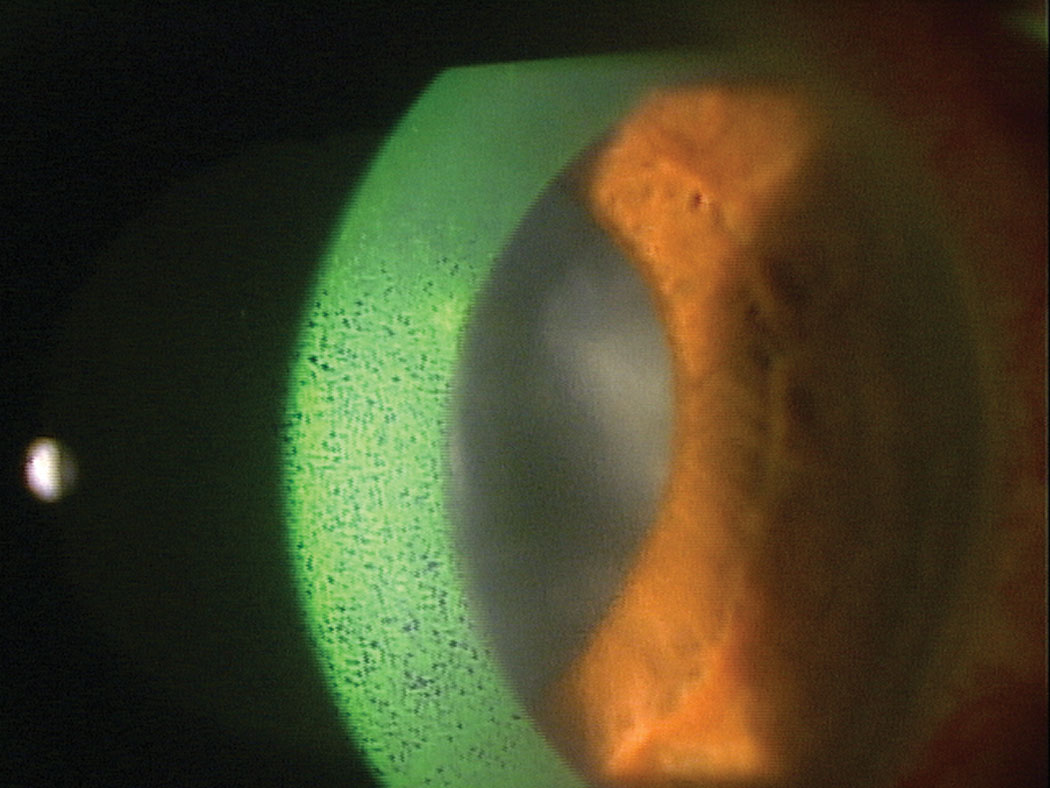 |
| Dry eye is the focus of most serum tear research that suggests AST concentrations as high as 50% could be safe and effective options to reduce signs and symptoms of DED. Click image to enlarge. |
Limited Roadblocks
Complications, contraindications and barriers to serum tear treatment are few. The regularity of blood draws requires serum tear candidates have a healthy tolerance for blood volume loss and venipuncture.4 The non-preserved nature of the drop means new bottles are opened each day and discarded within 16 hours.1 The lack of preservatives also increases the risk for infection and contamination; however, storage and usage protocols are intended to mitigate this as much as possible.1,6 Serum preparers and patient caregivers are also at risk of viral transmission, further underscoring the importance of donor serology screening.1 Secondary microbial keratitis has been reported in rare cases due to using tears in patients with a bacterial or fungal ulcer; therefore, corneal sterilization prior to initiation of treatment is essential.1,6,12
The literature notes that some rheumatoid arthritis patients have experienced scleritis or scleral melt following treatment with serum tears.1,4 Investigators hypothesize that circulating antibodies within serum eye drops may combine with corneal antibodies to form an immune complex, which may elicit this inflammatory response in rheumatoid arthritis patients.1
Furthermore, many patients successfully treated with serum tears experience relapse in symptoms if serum tears are fully discontinued.1,6
Despite their benefits, serum tears are not readily available to many patients. They must be produced at compounding pharmacies, eye banks or well-equipped ophthalmology offices.6 Insurance coverage is also varied, and patients could pay as much as $200 for a 30-day supply.6,11
Serum eye drops are an effective therapy for severe dry eye patients. The shared biochemistry with natural tears offers potential advantages over traditional therapies, including improved surface restoration, the avoidance of adverse reactions due to preservatives and the lack of antigenicity. Because of these possible benefits for patients, clinicians should consider adding serum tears to their dry eye toolkit for their moderate to severe dry eye patients.
Dr. McGinty-Tauren is a staff optometrist at the Battle Creek VAMC where she serves as residency program coordinator. She is also a fellow and committee member of the American Academy of Optometry.
Dr. Cornelius completed an ocular disease residency through the Battle Creek VAMC and has joined the practice of LO Eye Care.
| 1. Geerling G, MacLennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmology. 2004;88:1467-74. 2. Alio JL, Rodriguez AE, Ferreira-Oliveira R, et al. Treatment of dry eye disease with autologous platelet-rich plasma: a prospective, interventional, non-randomized study. Ophthalmol Ther. 2017; 6(2):285-93. 3. Hussain M, Shtein RM, Sugar A, Soong HZ. Long-term use of autologous serum 50% eye drops for the treatment of dry eye disease. 2014;33(12). 4. Pan Q, Angelina A, Marrone M, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2017;(2):1-46. 5. Wilson SE, Mohan RR, Hong J, et al. Apoptosis in the cornea in response to epithelial injury: significance to wound healing and dry eye. Adv Exp Med Biol. 2002;506(Pt B):821-6. 6. Shtein RM, Shen JF, Kuo AN, et al. Autologous serum-based eye drops for treatment of ocular surface disease. Ophthalmology. 2020;127:128-33. 7. American Academy of Ophthalmology. AAO Dry Eye Syndrome Preferred Practice Patterns. Elsevier;2018. 8. Celebi AR, Ulusoy C, Mirza GE. The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthlalmol. 2014;252(4):619-26. 9. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjogren’s syndrome. Br J Ophthalmol. 1999;83(4):390-95. 10. Ripa M, Jabbehdari S, Yazdanpanah G, et al. The role of multisystem disease in composition of autologous serum tears and ocular surface symptom improvement. Ocul Surf. 2020;18(3):499-504. 11. Badami KG, McKellar M. Allogeneic serum eye drops: time these became the norm? Br J Ophthalmology. 2012;96(8):1151-52. 12. Kim KM, Shin YT, Kim HK. Effect of autologous platelet-rich plasma on persistent corneal epithelial defect after infectious keratitis. Jpn J Ophthalmol. 2012;56:544-50. 13. Lee JH, Kim MJ, Ha SW, Kim HK. Autologous platelet-rich plasma eye drops in the treatment of recurrent corneal erosions. Korean J Ophthalmol. 2016;30(20):101-07. 14. Pandey AN. Management of severe dry eye: role of autologous serum eye drops. Int J Open Access Ophthal. 2017;2(2):1-3. 15. Krader CG. Topical hematopoietic therapy viable option for ocular surface disease. Ophthalmology Times. 2019 March;(3). 16. Kojima T, Ishida R, Dogru M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: A prospective randomized case-control study. Am J Ophthalmol. 2005;139(2):242-46. 17. Lemp M, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011;151(5):792-98. 18. Lee YK, Lin YC, Tsai SH, et al. Therapeutic outcomes of combined topical autologous serum eye drops with silicone-hydrogen soft contact lenses in the treatment of corneal persistent epithelial defects: a preliminary study. Cont Lens Ant Eye. 2016;39(6):425-30. |



