 Benzalkonium chloride (BAK)—a quaternary ammonium—is a highly hydrosoluble bipolar compound. BAK is both a preservative and a disinfectant—commonly found in leave-on skin antiseptics, hygienic hand wipes, nasal drops, floor cleaners, surgical instrument sterilization, air disinfectants and over-the-counter cold sore treatments. Since it is non-flammable and causes less skin irritation than alcohol, it has in fact become the disinfectant of choice in many healthcare settings. BAK is also non-staining and non-corrosive on metals. As a bipolar compound, BAK also works as a surfactant and is used in antistatic and emulsifying agents, conditioners and textile softeners. As a surface active agent, BAK is likely to interact with other surfactants in ophthalmic formulations—which could affect the its concentration in solutions and alter its preservative activity.1
Benzalkonium chloride (BAK)—a quaternary ammonium—is a highly hydrosoluble bipolar compound. BAK is both a preservative and a disinfectant—commonly found in leave-on skin antiseptics, hygienic hand wipes, nasal drops, floor cleaners, surgical instrument sterilization, air disinfectants and over-the-counter cold sore treatments. Since it is non-flammable and causes less skin irritation than alcohol, it has in fact become the disinfectant of choice in many healthcare settings. BAK is also non-staining and non-corrosive on metals. As a bipolar compound, BAK also works as a surfactant and is used in antistatic and emulsifying agents, conditioners and textile softeners. As a surface active agent, BAK is likely to interact with other surfactants in ophthalmic formulations—which could affect the its concentration in solutions and alter its preservative activity.1
BAK is a rapidly acting disinfecting agent with a moderately long duration of action. It is active against bacteria, some viruses, fungi and protozoa. Bacterial spores are considered to be resistant.2 BAK compromises cellular permeability by breaking down the membrane lipid bilayers, which induces leakage of the cellular contents.
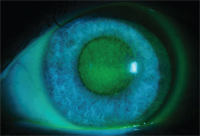
Chemical toxicity secondary to multiple topical antimicrobial medications.
BAK solutions are either bacteriostatic or bactericidal, depending on the concentration. Gram-positive bacteria are generally more susceptible to BAK than Gram-negative. Although biocidal activity is not greatly affected by pH, it can increase substantially at higher temperatures and with prolonged exposure.
A recent study has found that BAK—when used in less than lethal concentrations—resulted in a 256-fold increase in resistance of the bacteria Pseudomonas aeruginosa to the antibiotic ciprofloxacin, even though the bacterial colonies had not been previously exposed to the antibiotic.3
BAK as a Preservative
BAK is the most commonly used preservative in eye drops. Unfortunately, numerous studies have revealed deleterious corneal effects associated with BAK.4 These unwanted effects include destabilization of the tear film, death of corneal and conjunctival epithelial cells, morphological changes in the corneal epithelial cells and the reduction of the corneal epithelial barrier function.5
Symptoms of BAK toxicity and/or allergy include irritation and discomfort (foreign body sensation, itching or burning).6 Another severe side effect is chronic inflammation or the progressive development of fibrosis with higher risk of failure after glaucoma filtering surgery.5
The risk of side effects is likely dose dependent. Eye drops typically contain 0.002% to 0.01% benzalkonium chloride.1 Patients with chronic dosing of drops—including antibiotics, steroids, antiglaucoma drops and artificial tears—are more likely to develop complications from BAK (see photo). Compromised eyes may be less likely to recover from BAK toxicity; therefore, many doctors prescribe preservative-free formulations.
Despite the potential problems, preservatives are generally considered to be a good thing. They keep us safe from microbial contamination and extend the shelf life of products. They are an advantage for cost conscious consumers because they allow for multi-dose containers, which are significantly less expensive than single-dose bottles. It is therefore unlikely we will see the demise of preserved eye drops any time soon. Just don’t use too much of a good thing.
1. Liu J, Lu GW, Sandoval M, et al. Determinatin of benzalkonium chloride partition in micelle solutions using ultrafiltration method. AAPS PharmSciTech. 2009 December; 10(4):1216–23.
2. Lazzaro DR, Abulawi K, Hajee ME. In vitro cytotoxic effects of benzalkonium chloride on adenovirus. Eye Contact Lens. 2009 Nov;35(6):329-32.
3. Mc Cay PH, Ocampo-Sosa AA, Fleming GT. Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiology. 2010 Jan;156(Pt 1):30-8. [Epub 2009 Oct 8]
4. Kusano M, Uematsu M, Kumagami T, et al. Evaluation of acute corneal barrier change induced by topically applied preservatives using corneal transepithelial electric resistance in vivo. Cornea. 2010 Jan;29(1):80-5.
5. Baudouin C, Labbé A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010 Jul;29(4):312-34. [Epub 2010 Mar 17]
6. Hong J,Bielory L. Allergy to ophthalmic preservatives. Curr Opin Clin Immunol. 2009 Oct;9(5):447-53.


