Even though soft contact lenses dominate the market, gas permeable (GP) lenses remain essential for many patients, providing superior optics, durability, higher oxygen transmissibility and a lower propensity for surface deposits and microbial inoculations within the GP lens matrix. Modern specialty lenses, especially scleral-fit GP lenses, offer better outcome expectations for patients with a wide range of visual and clinical needs—from optical rehabilitation in patients with corneal irregularities to therapeutic protection in patients with dry eye and other ocular surface diseases.1,2 As such, global clinical trends demonstrate a growing rate of GP contact lens use.3-5 This finding especially holds true for scleral lenses specifically.3-5
While specialty lenses are becoming more popular, fitting them can sometimes intimidate clinicians who are less familiar with custom designs. This is primarily due to the perceived extensive chair time and potential patient discomfort with corneal GP lenses. However, as corneal GP technology advances, new lens designs—like the design of a scleral lens, for example—make it possible to improve initial lens comfort and reduce adaptation time.
The on-eye position and associated fitting characteristics of scleral lenses are heavily influenced by the corneo-conjunctival junction angle and scleral shape profile. Consequently, if a scleral GP cannot be customized to align with these anatomical features, fitting- and vision-related adverse reactions may develop.6 To make matters worse, slit lamp findings alone do not usually offer sufficient clinical clues for clinicians who are looking to take advantage of the latest lens customization concepts.
Fortunately, numerous technologies are available to help overcome some of these challenges and deliver optimal results. This article walks through five key steps clinicians should take to successfully complete a specialty contact lens fitting and explains how several newer diagnostic instruments can be clinically helpful for novices and veterans alike.
Strictly speaking, you do not need any of these devices to be successful with scleral lenses in a number of routine cases. But these tools will help you solve problems, save time and fine-tune results to give each patient the most customized experience possible. An important mark of expertise in all contact lens specialists is whether they use the best available tools of the trade and stay both well equipped and well versed in their product knowledge.
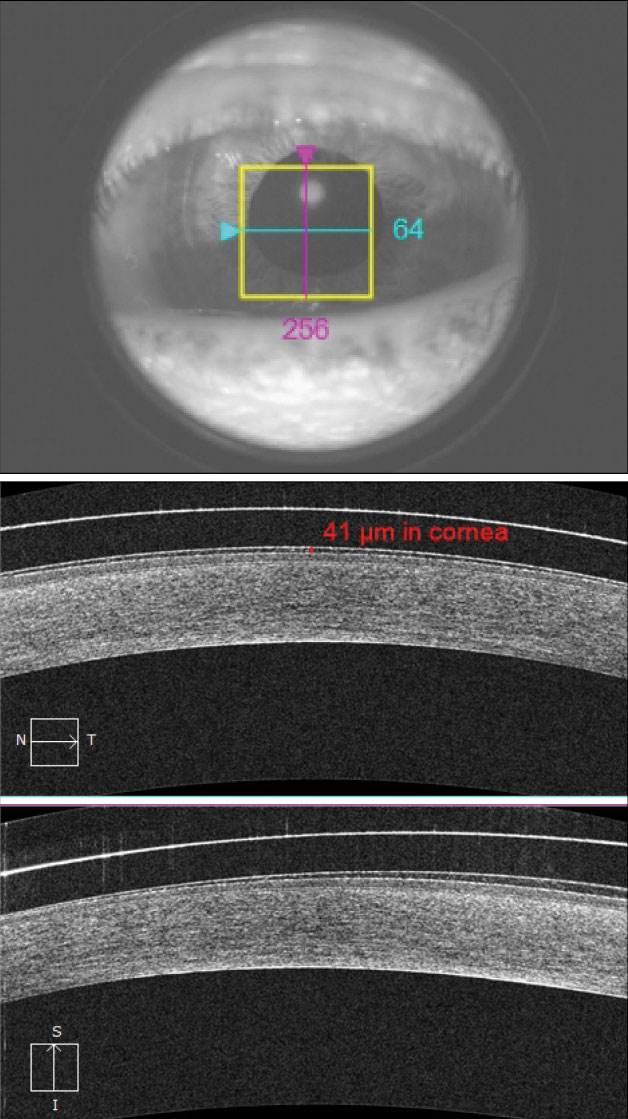 |
| Fig. 1. This hybrid lens shows a mild amount of corneal clearance (41μm). The patient needs an increased lens vault to properly compensate for the lens settling throughout the day. |
1. Measure the Vault
The long-term objectives of a contact lens fit are to meet metabolic requirements of the corneal tissue and reduce hypoxic risks. Compared with the vaulting abilities of corneal-fit GP lenses and soft lenses, a scleral lens’s ability to vault over the cornea helps avoid epithelial trauma and, in turn, improves comfort. Vaulting also allows the accumulation of tears to neutralize anterior corneal toricity and irregularities. The vaulting distance of a scleral lens, however, may act as a pathway that resists efficient oxygen permeation. Researchers tend to agree that long-term monitoring and using lens materials with high oxygen permeability (>100 Dk/l) are prudent practices when fitting scleral GP lenses.7-10
A team of researchers suggests three clinical criteria must be checked off to achieve maximum oxygenation under a scleral lens: (1) the lens must be constructed from a material with the highest Dk available, (2) it must have a maximum center thickness of 250µm and (3) it must be fit with no more than 200µm of clearance.7 Perhaps surprisingly, the third criterion appears to have the largest clinical significance; another group of researchers also concludes that tear vault has a bigger impact on corneal oxygen tension than lens Dk.9 To support previous findings, a follow-up study demonstrates that corneal oxygenation levels drop by as much as 30% when scleral vault increases from 200µm to 400µm.10
Despite the clinical importance of the recommended 200µm clearance guideline, this proposed fitting condition might be the most challenging to fulfill. This is largely due to the variability in tear lens measurement via slit lamp biomicroscopy. Research shows that a variance of up to 207µm may occur when estimating scleral lens vault using a slit lamp.11 Thus, if clinicians are expected to follow the 200µm clearance suggestion but must rely on slit lamp observations to guide vaulting modifications, patients may be exposed to undue risk of hypoxia.
A more precise and objective method of measuring corneal vault is anterior segment optical coherence tomography (AS-OCT). The technology’s caliper tool is accurate within 6µm, offering more precise clearance data in multiple locations under scleral lenses and other specialty lenses (Figure 1). Nonetheless, this technology comes with limitations; it assumes the measurement path travels from the refractive indices of air to cornea and does not currently account for the additional medium of contact lens material. Despite this, AS-OCT is still substantially more accurate and repeatable than slit lamp biomicroscopy.
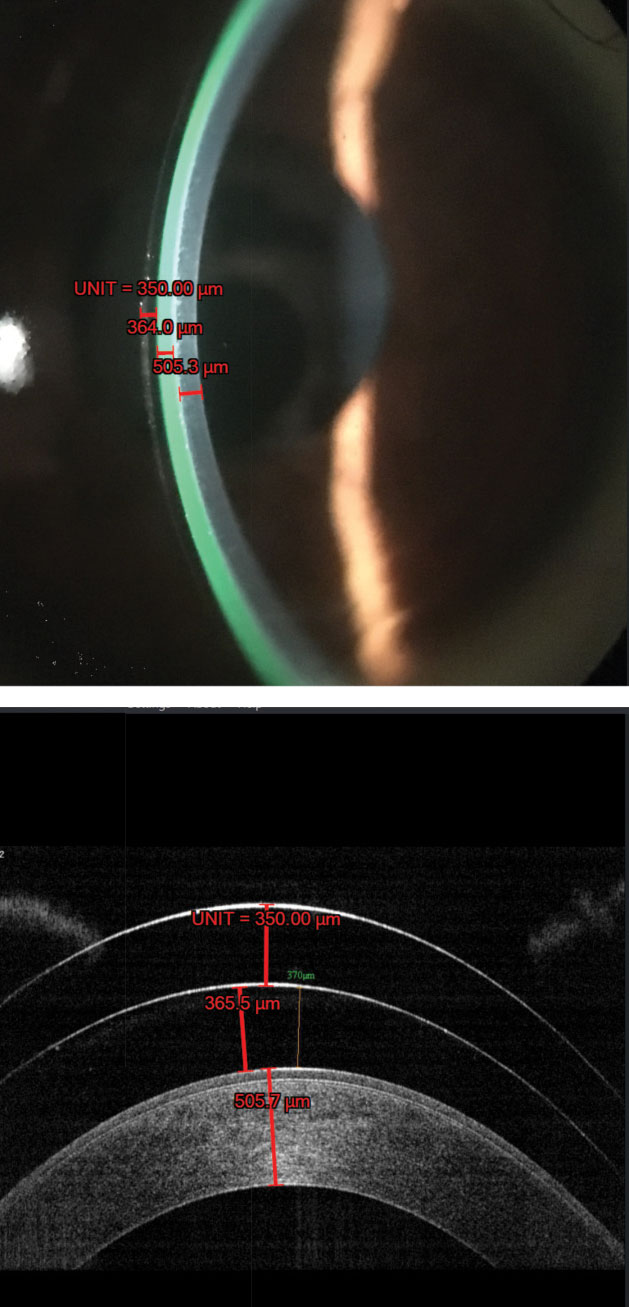 |
| Fig. 2. The ruler function in the AOS software calibrates itself by using the known lens center thickness value (i.e., 350μm) and then calculates the corneal clearance and thickness as shown on the imported digital image (top). After an OCT image of the same patient is uploaded to the software, the calibrated ruler function yields nearly identical measurements for corneal clearance and thickness (bottom). Image: John Gelles, OD |
If you do not currently have access to AS-OCT, new software that analyzes the ocular surface—AOS (Advanced Ophthalmic Systems)—may be worth pursuing as a cheaper alternative. While validation between AS-OCT and AOS measurements is currently limited, this Windows-based technology can analyze and enhance any digital image, whether it originates from an anterior segment camera or a smartphone.
This software package has various clinical grading scales for conjunctival redness, vascular status, punctate epitheliopathy and more. It also includes a specialty lens-fitting module that measures scleral lens clearance. Additionally, this software indirectly assesses the peripheral lens fitting relationship by grading vasculatures within selected areas under the haptic zone, which may further aid clinicians in deciding whether to incorporate customizable features, such as a back-toric haptic (Figure 2).
2. Perfect the Design
Even with a history of corneal GP non-adaptation, most patients can still comfortably wear modern hybrid lenses and scleral lenses. When patients present with a history of contact lens failure, clinicians typically place more emphasis on lens comfort than sight. For example, a patient with severe keratoconus but relatively clear corneas and otherwise normal ocular findings may be conditioned by their doctor to be content with a visual outcome of 20/40, provided their lenses are comfortable. Fortunately, technologies are available to help give patients comfort and optimal vision.
Despite improved fitting success with today’s hybrid lenses and sclerals, anatomical variations in the corneo-conjunctival junction angle and the scleral profile often affect the scleral lens fitting and visual outcome. As a result, scleral lenses may cause misalignment between the visual axis and the optical center, inducing lens instability and producing unintended higher-order aberrations (HOAs)—all of which can lead to unsatisfactory visual performances. Modern scleral lenses, however, have design-specific angular geometry built into the transition zone—new scleral lens designs tend to use a transition zone that assumes a tangential angular profile—which increase fitting stability because the scleral lens is able to better align with the anatomy of the corneo-conjunctival junction.
Recent studies show that scleral lenses become increasingly rotationally asymmetrical as the chord diameter on the ocular surface widens. Thus, incorporating back-toric in the haptic zone may improve both comfort and stability.6,12 To make the process easier, some scleral lens technologies can develop different haptic angles for right and left eyes in diagnostic sets.
If the patient’s vision remains below clinical expectations, even with enhanced centration and stability through the use of toric haptics, clinicians can perform a toric over-refraction to see if better visual acuity is attainable. If spherocylindrical over-refraction findings are clinically significant, incorporating front-toric optics into a lens with toric haptics will promote rotational stability, yielding a higher success rate.
Optical complexities can vary depending on individual case presentations, and some patients may continue to suffer from compromised visual function. A study suggests that the clinical benefits of modifying the front surface eccentricity of scleral lenses to 0.6 or 0.8 are analogous to adding wavefront-guided optics, whereby improvements in both high- and low-contrast visual acuity can be observed.13 Thus, it is worth inquiring about this new customization feature with lab consultants.
A Lens That Fits Like a Glove
EyePrint Prosthetics (EPP) introduced a manufacturing technology that aims to replicate the contours of a patient’s ocular surface by using an ophthalmic-friendly molding material that captures the exact impression of the anterior ocular anatomy. The resultant impression is shipped to a manufacturing lab, where one to two million data points are scanned and converted into computer-aided design images, producing precise scleral lens geometry.
Designing such a highly customized lens makes a wide range of clinical applications possible. EPP technology can accurately position itself on-eye, regardless of irregular conjunctiva-sclera angles or scleral elevations, such as pterygia, severe pingueculae and glaucoma blebs/shunts. |
3. Finesse the Fit
A recent study demonstrates that most eyes have some level of scleral asymmetry or irregularity.6 Using a corneo-scleral topographer to scan ocular surfaces at 16mm diameters—a commonly used scleral lens diameter length—researchers found that only 5.7% and 28.6% of evaluated eyes had scleral profiles resembling spherical and regular toric shapes, respectively.6 These findings imply that, when using a 16mm mini-scleral lens, haptic misalignment could potentially be observed in up to 71.4% of eyes even if a standard toric haptic design is used.
Misaligned scleral lens haptics may lead to discomfort, air bubble formation and debris trapped in the lens reservoir. Significant debris entrapment can result in visual fogging, which drastically decreases satisfaction. To combat this major barrier, clinicians must perfectly match scleral lens profiles, limbal transition angles and conjunctiva-sclera angles in all meridians.
This is where a corneo-scleral topographer comes in handy. It can capture circumferential ocular surface elevation data at a specified chord diameter and guide the manufacturing process to produce the appropriate amount of haptic toricity and the desired total lens sagittal depth. In addition, new software can compute the height and size of a lesion, such as a pinguecula, and create a focalized vaulting area under the scleral lens to best match the ocular surface profile.14 This software can greatly reduce the chair time traditionally associated with designing a scleral lens notch.
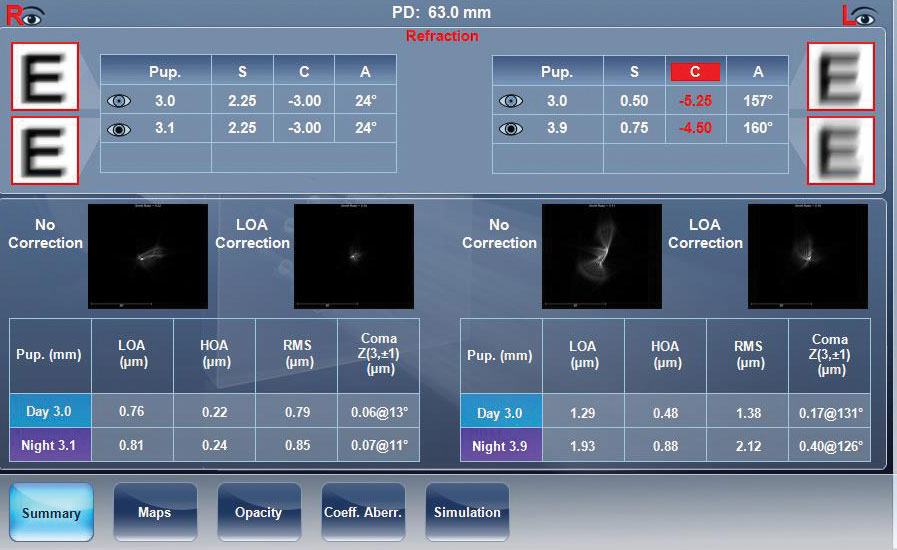 |
| Fig. 3. In this wavefront over-refraction performed on a severe keratoconus patient who wears scleral lenses, the 3mm pupil measurement zone shows a -3.00D residual cylinder OD and a -5.25D residual cylinder OS. |
4. Sharpen the Optics
The customization strategies discussed thus far help enhance specialty lens fitting outcomes. Some patients, however, will continue to experience reduced vision due to the presence of residual HOAs. Therefore, measuring these HOAs will assist you in finding ways to address continued visual challenges.
To account for the optical summation of both anterior and posterior corneal profiles, diagnostic instruments capable of registering corneal data points beyond anterior corneal shape can further elucidate sources of visual disturbance. Scheimpflug imaging devices, such as the Pentacam (Oculus), can analyze anterior and posterior corneal profiles through a rotating mirror that travels in an arc around the eye and produces a 3D biometry of the eye.
Many will find that accounting for posterior corneal contributions is sufficient in obtaining desired clinical outcomes. However, using a wavefront aberrometer to precisely map HOAs will give you more data to consider when fine-tuning lens specs. Popular devices include the OPD-III (Nidek), KR-1W (Topcon) and iTrace (Tracey Technologies).
Some newer devices combine multiple diagnostic capacities to render a comprehensive analysis of the anterior segment. The VX130 (Visionix) is equipped with the functionalities of a Shack-Hartmann wavefront aberrometer, an autorefractor, a Scheimpflug-enabled corneal tomographer, an optical pachymeter and a non-contact tonometer. This single instrument allows clinicians to visualize the differential spatial arrangements between the anterior and posterior cornea and provides a wavefront over-refraction to help guide contact lens modifications (Figure 3).
In some cases, clinicians can compensate for residual HOA contributions with a spherocylindrical over-refraction, proving that wavefront aberrometry refraction over a specialty lens can be helpful during the initial fitting and subsequent follow-up. Nonetheless, depending on the magnitude of posterior optical complexities and the on-eye position of the lens, additional anti-aberration mechanisms could be required.
Researchers and manufacturers have already found success when incorporating wavefront-guided corrections into scleral lenses.15,16 Thus, capturing HOA data will help guide the process of designing specialty contact lenses for your more challenging patients. As lab manufacturers further refine their own proprietary algorithms, patients will continue to benefit from the use of these advanced technologies.
 |
Fig. 4. Eyecare Live allows patients to send ocular images securely to their eye doctors via telemedicine. Before a follow-up, digital interactions with their doctor give patients the chance to ask questions and express concerns while adapting to their newly prescribed specialty lenses. |
5. Keep in Touch
Constructing the perfect specialty lens for each patient requires a high level of clinical expertise, in addition to patient motivation and compliance. Although perhaps a controversial subject, telehealth applications can greatly benefit patient-doctor communication when used appropriately.
As is often the case with specialty contact lens evaluations, patients travel long distances to seek the expertise of clinicians who have a limited amount of time. Consequently, clinicians must identify ways to achieve the best clinical results within a limited number of office visits.
One solution may be a doctor-to-patient telehealth platform called Eyecare Live. The clinical objective is to use this platform to check in with specialty contact lens patients prior to their follow-up exams and complete non-office-related tasks online. From the comfort of their homes, patients can communicate questions and submit lens-related ocular images for analysis (Figure 4). This process aims to improve patient compliance and motivate patients to continue their lens adaptation efforts for a greater chance of fitting success.
A Win-Win Scenario
A specialty lens fitting can significantly improve a patient’s quality of life if done successfully. However, selecting the most appropriate lens requires a high level of clinical expertise and the right pieces of equipment. Rather than focus on traditional specialty lens designs, clinicians should update their fitting strategies based on the most recent evidence-based data available, selectively adopting new diagnostic instruments and exploring alternate interfaces that can enhance clinical interactions and improve standards of care. As a result, clinicians and patients alike will have better experiences, feel more satisfied and see successful outcomes.
Dr. Chang is the Director of Cornea Specialty Lenses at Wills Eye Hospital and the Director of Clinical Services at TLC Vision. He is also the host of a new podcast series called “Chang Reaction.”
Dr. Sonsino is a partner in a specialty contact lens and anterior segment practice in Nashville, Tenn. He is a diplomat of the Cornea and Contact Lens section of the American Academy of Optometry and the past chairman of the Cornea and Contact Lens section of the American Optometric Association.
1. Barnett M, Mannis MJ. Contact lenses in the management of keratoconus. Cornea. 2011;30(12):1510-6. 2. Bavinger JC, DeLoss K, Mian SI. Scleral lens use in dry eye syndrome. Curr Opin Ophthalmol. 2015;26(4):319-24. 3. Morgan PB, Woods CA, Tranoudis IG, et al. International contact lens prescribing in 2016. Contact Lens Spectrum. 2017;32(1):30-5. 4. Morgan PB, Woods CA, Tranoudis IG, et al. International contact lens prescribing in 2017. Contact Lens Spectrum. 2018;33(1):28-33. 5. Nichols JJ. Contact lenses 2017. Contact Lens Spectrum. 2018;33(1):20-5,42. 6. DeNaeyer G, Sanders D, van der Worp E, et al. Qualitative assessment of scleral shape patterns using a new wide field ocular surface elevation topographer. J Cont Lens Res Sci. 2017;1(1):12-22. 7. Michaud L, van der Worp E, Brazeau D, et al. Predicting estimates of oxygen transmissibility for scleral lenses. Cont Lens Anterior Eye. 2012;35(6):266-71. 8. Jaynes JM, Edrington TB, Weissman BA. Predicting scleral GP lens entrapped tear layer oxygen tensions. Cont Lens Anterior Eye. 2015;38(1):44-7. 9. Compañ V, Aguilella-Arzo M, Edrington TB, et al. Modeling corneal oxygen with scleral gas permeable lens wear. Optom Vis Sci. 2016;93(11):1339-48. 10. Giasson CJ, Morency J, Melillo M, et al. Oxygen tension beneath scleral lenses of different clearances. Optom Vis Sci. 2017;94(4):466-75. 11. Brujic M. Poster Presented at the Global Specialty Lens Symposium, Las Vegas, NV, January, 2016. 12. Visser ES, Visser R, Van Lier, HJ. Advantages of toric scleral lenses. Optom Vis Sci. 2006;83(4):233-6. 13. Hussoin T, Le HG, Carrasquillo KG, et al. The effect of optic asphericity on visual rehabilitation of corneal ectasia with a prosthetic device. Eye Contact Lens. 2012;38(5):300-5. 14. Achenbach P, DeNaeyer G, Sanders D. Simplifying Scleral Lens Fitting in The Presence of Localized Elevations with a New Corneo-Scleral Topography System: Notches and Lifts. Poster Presented at the American Academy of Optometry, Chicago, IL, October, 2017. 15. Slater DJ, Lay B, Sindt CW. Using corneal elevation specific technology to anti-aberrate a contact lens. Invest Ophthalmol Vis Sci, 2016;57(12):1489. 16. Sabesan R, Johns L, Tomashevskaya O, et al. Wavefront-guided scleral lens prosthetic device for keratoconus. Optom Vis Sci. 2013;90(4):314-23. |




