 | |
| Fig. 1. Optimal fluorescein pattern of silicone hydrogel mini-scleral contact lens. | |
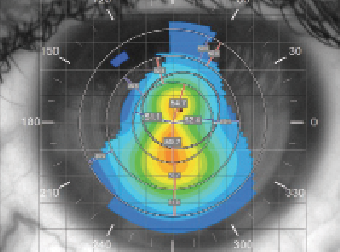 | |
| Fig. 2. Corneal topography of the left eye reveals advanced keratoectasia. |
Corneal collagen crosslinking (CXL) available internationally for over a decade and now newly approved in the United States, is the only surgical procedure capable of slowing down or stopping the progression of keratoconus and secondary keratectasia after laser in-situ keratomileusis (LASIK) and photorefractive keratectomy (PRK).1,2 CXL is also considered by many to be a vision-saving procedure, especially when performed on younger patients with the goal of preventing the need for future keratoplasty. Research suggests CXL may have beneficial visual and optical effects, as evidenced by the reduction in corneal steepness and improvement in uncorrected visual acuity and best corrected visual acuity (BCVA).3,4 Despite these impressive outcomes, however, in many cases corrective lenses are still necessary to achieve the best possible vision following CXL.
Specialty soft contact lenses in particular continue to gain popularity among eye care practitioners due to advancements in lens materials and lathing technology.5 One such example is the silicone hydrogel mini-scleral (SHmS): a large 17mm diameter lens that vaults the limbus and central cornea with minimal bearing on the corneal apex to rest on the patient’s sclera. The lens’ central thickness varies from 0.5mm to 0.6mm and is responsible for the neutralization of corneal irregularity and the creation of a regular front refractive surface. Scleral support also results in the formation of a true aqueous tear lake underneath the lens, which enhances the ability to correct irregular astigmatism, similar to gas permeable (GP) scleral lenses (Figure 1). One major indication for this type of lens is visual rehabilitation following CXL, with the goal of the fitting being to provide a stable, distortion-free refractive surface and minimize interference between the recovering ocular surface—especially the epithelium—and the contact lens.6 Examples of custom soft contact lenses for keratoconus include the NovaKone (Alden Optical), Flexlens Tricurve (X-Cel Specialty Contacts), and Eni-Eye Soft-K lens (Acculens).
Semi-scleral soft lenses can also be used as therapeutic bandage lenses following refractive or ocular surface reconstructive surgery, especially in eyes with high corneal toricity or steeper than average curvature, since a higher sagittal depth helps stabilize lens fit and reduce excessive lens movement. Examples include the Kontur (Kontur Kontact Lens) or T74/85 (David Thomas Contact Lenses); however, the application of these designs is limited entirely to therapeutic purposes due to their inability to correct irregular astigmatism.
So, with the CXL era finally about to begin in the US, let us consider whether the approach to contact lens fitting shortly after CXL should be different from what we consider a standard of care for the irregular cornea patient through a series of case reports.
1. Standard Procedure
A 29-year-old woman with a diagnosis of surgically-induced keratectasia was referred in for a specialty contact lens fitting due to decreased vision in her left eye for the last nine months. She reported that she had undergone LASIK for moderate myopia 12 years prior. The patient’s unaided visual acuities were 20/80 OD and 20/200 OS, and her refraction was +1.25/-3.75x140 OD and -5.00/-5.00x170 OS with best-corrected visual acuity (BCVA) of 20/30 and 20/80, respectively. Corneal topography revealed advanced keratectasia predominantly in the left eye (as compared with her right) with maximum keratometry (Kmax) values of 55.4D OD and 59.7D OS (Figure 2). The patient was subsequently evaluated by a cornea specialist and scheduled to undergo an epithelium-off corneal collagen crosslinking procedure to halt further progression. She was also given temporary spectacles with partial astigmatic correction in the right lens and plano correction in the left to wear following the surgery. Two weeks after, the patient underwent CXL in her left eye.
 | |
| Fig. 3. Well fitted silicone hydrogel mini-scleral lens. Proper allocation of the fenestration holes (at 3 and 9 o’clock) indicates absence of lens rotation. | |
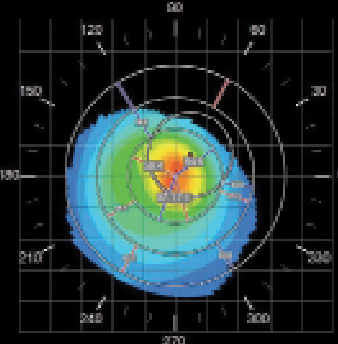 | |
| Fig. 4. Corneal topography of the right eye demonstrating a classic keratoconic nipple cone appearance. | |
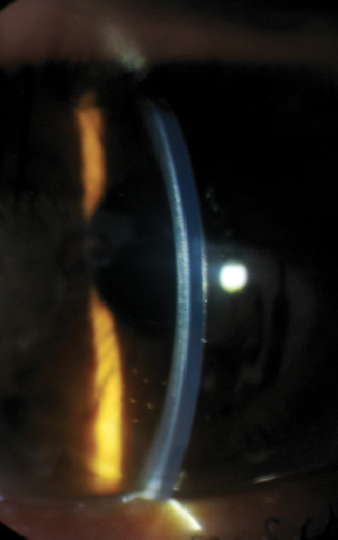 | |
| Fig. 5. This image represents 6.50mm lens with an excessive central touch and minimal mid-peripheral clearance. Note the presence of the anterior corneal haze. The lens thickness is 600μm. |
Five weeks later, following complete epithelial healing and the discontinuation of all steroid medications, the patient’s left cornea exhibited no signs of epithelial hypertrophy or superficial punctate staining. As such, she was scheduled for a contact lens fitting and given a front toric, prism-ballasted version of the SHmS lens with a base curve of 7.3mm and the power of -3.75/-2.25x180. The lens was manufactured in a Filcon V3 silicone hydrogel material (Definitive 74, Contamac). At the dispensing visit, the patient’s BCVA was 20/30 OS, with a minor complaint of ghost images. She was instructed to increase lens wearing time gradually, and was scheduled for a follow-up appointment in the next 10 days.
During the follow-up appointment, a slight reduction in visual acuity (i.e., 20/50) was noted, but the patient reported noticeable improvement in vision clarity and her ability to perform daily tasks, as well as good lens tolerance and average lens wear of nine hours a day. An examination revealed the presence of a well-centered lens with about 1mm of on-blink movement (Figure 3). An overrefraction of plano/-1.50x175 was able to improve her vision to 20/25 OS and significantly reduce the amount of ghost images seen. A new lens with an updated power was ordered; three weeks later, the patient was able to wear the revised lens up to 10 hours a day with stable contact lens-corrected vision. As a result of successful restoration of vision in the treated eye, the patient underwent an uneventful CXL procedure eight weeks later for her other eye.
2. Progression Protection
A 29-year-old male construction worker was diagnosed with bilateral keratoconus (OS>OD) 12 years prior to presenting to the clinic. He reported he had undergone penetrating keratoplasty in his left eye in 2011; however, shortly after the procedure, the corneal transplant had begun to show signs of endothelial rejection, which eventually led to scarring and reduced vision. Numerous GP lens fits had been attempted but the patient had continued to demonstrate a severe intolerance to rigid lenses, especially while outdoors. In the last three years, he had successfully managed using a soft lens made for keratoconus (Soft-K, Soflex) with parameters BC 7.00, diameter 14.20 an power -8.00. Additionally, he had been able to achieve a visual acuity of 20/40-. Unaided vision in his left eye was 20/400.
However, in early 2014 his right eye had begun to exhibit signs of keratoconus progression along with a consecutive decrease in BCVA. A new attempt to fit a GP lens also failed. The patient was referred to a cornea clinic for the possible necessity of CXL, who directed him to delay the procedure due to borderline corneal thickness of 408μm. He was scheduled for a follow up in six months but later the same year, the patient’s best-corrected vision dropped to 20/60, with marked steepening noted on corneal topography. To halt further deterioration of vision, an epithelium-on CXL procedure was performed. The postoperative recovery was uneventful, and four weeks later the patient had been scheduled for contact lens fitting.
An examination at the time of the contact lens fitting revealed the following findings: manifest refraction of -5.00/-6.50x125 VA 20/150 OD and -4.50/-7.50 x 110 VA 20/70 OS. Central keratometry readings of the right eye were 56.0/57.4D with a Kmax value of 62.5D (Figure 4). A slit lamp examination revealed a faint anterior stromal haze with an intact corneal epithelium (Figure 5). Fit assessment using the old right contact lens demonstrated an excessive rocking movement of the lens with fluting edges upon blink. Attempts to refit the patient’s right eye with a steeper Soft-K lens did not provide sufficient visual improvement (BCVA of 20/60). Instead, the patient was selected for a trial fitting with a SHmS lens made from Definitive 65 (Filcon V4) rather than the practice’s default material (Filcon V3, Contamac).
Filcon V4 is a polymer with a higher silicone content than its predecessor, Filcon V3. Other than the high oxygen permeability (Dk = 62) previously mentioned, the Filcon V4 material also possesses the highest modulus of elasticity in the family of lathable silicone hydrogels (MPa = 1.0). Typically, lenses with a higher modulus are stiffer and will correct the underlying corneal irregularly more effectively, while those lenses with a lower modulus will simply drape over the cornea. Fitting of contact lenses with a higher modulus may be advantageous in terms of vision correction success, since lenses made of these materials help mask the entirety of corneal astigmatism and eliminate the need for a toric design. On the other hand, wear of stiffer lenses can lead to edge fluting, tarsal irritation and mechanically-induced allergy. In these cases, additional adjustment of the peripheral curves is often required for successful lens wear.
This patient was fit into a spherical version of the SHmS lens with a base curve of 6.5mm, “steep” peripheral curve, diameter of 17mm and central lens thickness of 0.6mm (Figure 5). The lens power was -12.5D. Four fenestration holes were added at the lens limbal zone to increase oxygen delivery and tear mixing.
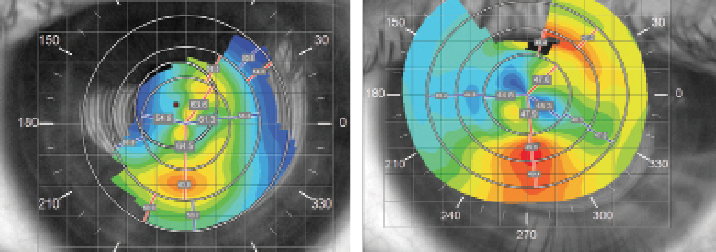 | |
| Fig. 6. (A) pre-treatment corneal topography of the right eye; and (b) topography of the same eye after the resolution of corneal hydrops. Note 14D flattening of Kmax. |
Vision upon dispensing was 20/50. At the one-month follow-up appointment, the patient reported good lens tolerance with 12 to 14 hours a day of lens wearing. Visual acuity had decreased to 20/60, but improved back to 20/40- with overrefraction of +2.50/-1.50x050. The patient’s central cornea exhibited trace central superficial punctate keratopathy, which appeared to relate to corneal apex bearing. A new lens with base curve of 6.4mm, similar peripheral geometry and a power of -10.5D was dispensed. Overrefraction demonstrated similar residual astigmatism findings of +0.50/-1.50x55. The patient was advised to wear polycarbonate spectacles for correction of residual astigmatism and ocular protection. Final visual acuity of the right eye improved to 20/30-. No significant corneal staining or corneal neovascularization were present at three-month follow-up. Current daily lens wearing time stands at 12 hours a day with the occasional use of lubrication agents for comfort.
3. Hydrops Hijinks
Successful use of full-size scleral lenses after corneal crosslinking has also been previously reported in the literature.7 Regular sclerals offer the advantage of minimal mechanical interaction with the treated zone and help promote ocular surface healing and provide optical benefits.
In this third case report, a 19-year-old male with advanced keratoconus OS and documented progression underwent an epithelium-off corneal crosslinking procedure in May 2013. Prior to the surgery, his maximum corneal steepness was 64.5D with an unaided vision measurement of 20/800 (Figure 6a). Unfortunately, no pre-treatment refractive data was available for this patient but postoperative recovery was positive for hypertrophic epithelium formation and delayed healing, which was treated using antibiotics and a bandage contact lens during the course of four weeks. At week five, the patient presented back to the clinic with complaints of significant pain and cloudy vision OS. His vision was reduced to CF at three feet and severe stromal edema with ruptures in Descemet’s membrane was observed (Figure 7).
The patient was treated for corneal hydrops with a five-week course of topical steroids, in combination with antibiotic coverage during the first seven days and hyperosmotic eye drops. After the patient’s cornea cleared up and the edema diminished, the patient was referred for a contact lens fitting.
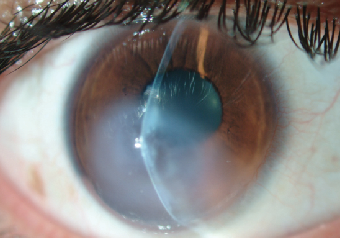 | |
| Fig. 7. Corneal hydrops four weeks after performance of the CXL procedure in the eye with delayed ocular surface healing. | |
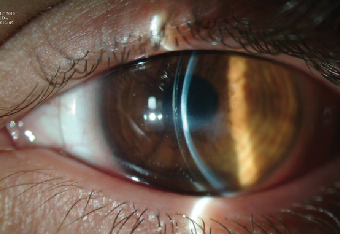 | |
| Fig. 8. A properly fitted 18.50mm scleral lens on the eye with resolved post-CXL corneal hydrops. Note the scar-induced central corneal flattening. | |
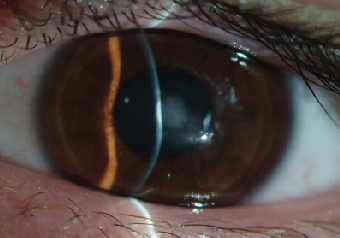 |
|
| Fig. 9. Dense central corneal scar after an episode of microbial keratitis. We assume that the infection was propagated by the unauthorized resumption of GP lens wear three weeks after uneventful CXL. |
|
A slit lamp examination revealed a stromal scar of moderate density at the inferior pupillary margin, trace punctate keratopathy of the inferior cornea, signs of meibomian gland dysfunction and floppy lid syndrome. Tear break-up time (TBUT) values were also low at seven to eight seconds. Corneal topography demonstrated a highly irregular corneal surface with significant flattening of the center and (Kmax value of ~50D) as compared to pre-treatment findings (Figure 6b). Considering the patient’s complex corneal geometry and decompensated ocular surface, we decided to manage this patient with a 18.5mm GP scleral lens. Incorporating the advances of reverse geometry technology, we were able to design a lens with a flat enough base curve to more precisely follow an oblate central cornea (Figure 8). The patient’s lens-corrected visual acuity was 20/40, and, after a short adaptation period, he was able to achieve day-long uncomplicated lens wear. At his one-month follow-up, his BCVA was unchanged, the corneal epithelium was intact and his TBUT values had improved to 15 seconds.
Keep in Mind
Though CXL is considered a potentially vision-saving procedure in many cases, it is not free of complications. The most common side effects are delayed epithelial healing, longstanding SPK, noninfectious infiltrates and stromal edema with scarring (i.e., corneal hydrops) as well as minimal reduction in endothelial cell count secondary to UV irradiation damage.8 Despite the ocular surface complications attributed to crosslinking, however, the risks associated with corneal transplantation surgery still significantly outweigh those of CXL.
Currently, there are no clear recommendations regarding when it’s safe to begin or resume contact lens wear after corneal collagen crosslinking. Post-CXL corneal recovery can fluctuate over time. Crosslinking research with data on long-term follow-up has demonstrated that corneal thickness, as well as central keratometry, follows a dynamic curve in the first six months.9,10 While patients following an epithelium-on procedure may be fitted in a time frame of one to two weeks, patients who undergo the epithelium-off treatment may need a significantly longer recovery time and may also experience a higher rate of corneal healing-related complications.
Though the epithelial defect typically closes four to seven days after the procedure, continuous epithelial remodeling followed by the modification in the arrangement of stromal collagen fibers and corneal nerve proliferation may be seen over several months. Consequently, it is imperative that healing is achieved prior to lens fitting to avoid mechanical disruption. These changes are also reflected in the further flattening and regularization of the central cornea, and may often lead to improved spectacle-corrected visual acuity and more success with contact lenses. For epithelium-off patients, we recommend resuming contact lens wear five to six weeks after the procedure, similar to post-PRK contact lens fitting indications.11
In the first months following CXL, the cornea may exhibit a thinner epithelial profile with decreased quality of adherence between the epithelial layers. These changes may cause a higher corneal vulnerability to contact lens-induced mechanical trauma. As such, fitting of traditional GP lens designs may prove problematic shortly after CXL; one of the most frustrating cases I have encountered recently is that of a 17-year-old male with advanced keratoconus (Kmax of 63D), who resumed wearing his old GP lenses three weeks after an epithelium-off procedure. One week later, he was admitted to the clinic with a complaint of significant eye pain, light sensitivity and blurred vision in the treated eye. Exam revealed dense infiltrative ulcerative keratitis at the center of the left cornea. After being on fortified cefazolin and gentamicin for almost two weeks, the ulceration resolved but left him with a dense corneal scar and best-corrected vision of 20/50 (Figure 9).
One study reports that patients fit with GP lenses three months after the procedure demonstrated evidence of epithelial cell stress with an increase in superficial epithelial cell size and a decrease in basal epithelial cell density.13 These findings are also accompanied by a decrease in corneal sub-basal nerve plexus density. The other area of concern regarding GP lens wear after CXL is that contact lens-induced mechanical irritation may lead to inflammation and consecutive keratocyte loss in the anterior stroma, in addition to apoptosis inflicted by UV irradiation.14
In summary, scleral and silicone hydrogel mini-scleral lenses in particular continue to possess clinical importance in today’s specialty contact lens practice. The use of these new lens designs may become a preferred alternative shortly after CXL. In addition to providing successful visual rehabilitation, they minimize contact lens influence on epithelial remodeling and allow uncomplicated ocular surface recovery after the procedure.
1. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135:620-7.
2. Hafezi F, Kanellopoulos J, Wiltfang R, Seiler T. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2007; 33:2035–40.
3. Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet A corneal collagen cross-linking for keratoconus in Italy: the Siena Eye Cross Study. Am J Ophthalmol. 2010; 149:585–93.
4. Greenstein SA, Fry KL, Hersh PS. Corneal topography indices after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011; 37:1282–1290
5. Fernandez-Velazquez FJ. Kerasoft IC compared to Rose-K in the management of corneal ectasias. Cont Lens Anterior Eye. 2012;35(4):175-9.
6. Severinsky B, Wajnsztajn D, Frucht-Pery J. Silicone hydrogel mini-scleral contact lenses in early stage after corneal collagen cross-linking for keratoconus: a retrospective case series. Clin Exp Optom. 2013;96(6):542-6.
7. Visser ES, Soeters N, Tahzib NG. Scleral lens tolerance after corneal cross-linking for keratoconus. Optom Vis Sci. 2015;92:318-23.
8. Wajnsztajn D, Strassman E, David Landau D, Frucht-Pery J. Ocular surface-related complications after corneal crosslinking for keratoconus. 19th Congress of ESCRS, Vienna, Austria, 2011.
9. Greenstein SA, Shah VP, Fry KL, Hersh PS. Corneal thickness changes after corneal collagen crosslinking for keratoconus and corneal ectasia: One-year results. J Cataract Refract Surg. 2011; 37:691–700.
10. Greenstein SA, Fry KL, Bhatt J, Hersh PS. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia: Scheimpflug and biomicroscopic analysis. J Cataract Refract Surg. 2010; 36:2105–14.
11. Woodward MA, Randleman JB, Russell B, et al. Visual rehabilitation and outcomes for ectasia after corneal refractive surgery. J Cataract Refract Surg. 2008;34:383–8.
12. Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. Epithelial and stromal remodeling after corneal collagen cross-linking evaluated by spectral-domain OCT. J Refract Surg. 2014;3:122-7.
13. Sehra SV, Titiyal JS, Sharma N, Tandon R, Sinha R. Change in corneal microstructure with rigid gas permeable contact lens use following collagen cross-linking: an in vivo confocal microscopy study. Br J Ophthalmol. 2014;98:442-7.
14. Kallinikos P, Efron N. On the etiology of keratocyte loss during contact lens wear. Invest Ophthalmol Vis Sci. 2004;45:3011–20.


