Scleral contact lenses have changed the lives of many of my patients, enabling them to see clearly while wearing a comfortable lens option. We are so fortunate to have several scleral lens designs (including front surface toric lenses for residual astigmatism, toric peripheral curves for eyes with great-than-average scleral toricity, reverse geometry and multifocal) available to us.1,2
These lenses are a fantastic choice for patients who have irregular corneas and glaucoma, and even more so if ocular surface disease (OSD) compromises the ocular surface in such patients. In fact, I have used them for many years on individuals with lid-induced OSD (Figure 1) and I would like to share a few clinical pearls for fitting scleral lenses in such instances. The lenses and designs mentioned are just a few of many possible solutions that practitioners have available today.
Case 1:
Bandage Be Gone!
A 74-year-old Caucasian male was referred to me by a corneal specialist for a contact lens fitting of the right eye. He complained of dryness in the morning and wore a soft bandage contact lens extended wear OD. Past history included an eyelid tumor OD; status post resection with lagophthalmos and corneal exposure. Corneal scarring was present OD and pseudophakia was present OU.
A scleral lens to manage the ocular surface and provide improved vision was discussed with the patient. If he was not successful with scleral lenses, the plan was to perform a penetrating keratoplasty OD. Even after a penetrating keratoplasty, a contact lens would be indicated to treat the ocular surface; a tarsarrophy would be the last resource, if needed.
• The exam. The patient’s medical history was significant for diabetes. Systemic medications included aspirin, glyburide, metformin, multivitamin and omega-3 fish oil. Visual acuity without correction was 20/400 OD (improved to 20/100 with pinhole) and 20/20 -2 OS. Manifest refraction of -2.25+3.25 x 070 OD provided 20/100. Simulated keratometry readings with topography OD: 48.49 @ 013 / 39.20 @ 103. Irregular astigmatism was present OD.
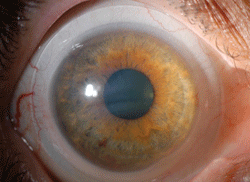
| |
| Fig. 1. Scleral lens used in ocular surface disease. |
Examination revealed ocular rosacea OU and a notch in the right upper eyelid with an absence of a portion of the eyelid from the eyelid resection. I also noted a stable punctal plug on the right lower eyelid, as well as a corneal scar extending from 11:00 to 5:00 with extension into the visual axis and neovascularization from 11:00 to 5:00 extending into the visual axis OD. I observed no epithelial defect. The anterior chamber was deep and quiet, and intraocular pressures (IOPs) were normal OU. Moreover, the posterior chamber intraocular lens (IOL) OU were stable and the optic nerve, macula and peripheral retina were all within normal limits.
• The fit. Once I was able to remove the bandage contact lens for the right eye without any complications, I fit a scleral contact lens to treat his ocular surface and irregular astigmatism.
Based on corneal topography measurements and visual ocular shape, the initial diagnostic lens selected was an Accu Lens Maxim scleral lens in Boston XO2 material (Bausch + Lomb) with a 16.5mm diameter. Lens parameters were OD 45.00D base curve (BC), 16.5-mm overall diameter (OAD), 9.50mm optical zone diameter (OZD), -4.00D power, sag 4.88. excessive central and peripheral vault was present with this lens.
The next diagnostic lens was OD 41.00D BC, 16.5mm OAD, 9.50mm OZD, plano power, sag 4.63. This lens had 100µm excessive vault centrally with peripheral blanching 360 degrees. Based off of the second diagnostic lens, lens parameters were OD 40.50D BC, 16.5mm OAD, 9.50mm OZD, +3.25D power, sag 4.55 flatter peripheral curves. The scleral lens fitting was successful, with good initial fit, vision and comfort and the patient had no trouble with insertion and removal.
• The result. Best-corrected visual acuity (BCVA) OD was 20/40-2. At the scleral lens dispensing and training appointment, the patient reported good vision and comfort with the lens. His visual acuity remained stable at 20/40-2, and he completed the insertion and removal training.
I advised him to discontinue the soft bandage contact lens (both day and night), but informed him to restart nighttime bandage contact lens wear if any dryness or irritation occurred during the day. I also prescribed lubricant ointment to be used at nighttime. No antibiotics were prescribed.
Two weeks later at the follow-up visit, the patient reported good vision and improved comfort with the scleral lens worn during the day. He was not using the bandage contact lens at night and had no complaints of dry eyes either day or night. The patient’s BCVA remained at 20/40-2 OD with no over-refraction.
At the time of the examination, he had been wearing the lens for five hours, but on average reported wearing the lens for up to 14 hours. He was using Clear Care (Alcon) and non-preserved sodium chloride 0.9% inhalation solutions.
Scleral lens fit of the right eye demonstrated good central apical clearance. I noted less clearance present nasal and temporal; however, the lens vaulted the entire cornea. Mild peripheral blanching was present in the nasal quadrant only from 2 o’clock to 4 o’clock associated with a nasal pinguecula. Conjunctival blanching may be due to a landing that is too flat or too steep. If blanching is under the entire area of the scleral lens, the landing may need to be increased by increasing the lens diameter. If blanching is under the scleral lens edge, it may cause conjunctival staining and hypertrophy over time. A notch could be considered; however, because the pinguecula was mild, it was not indicated. I did not observe any tear debris.
Upon removal of the lens, the eyelid appeared unchanged and the cornea demonstrated trace inferior punctate epithelial keratopathy without microcystic edema in either eye. I saw no evidence of a conjunctival impression ring, so I instructed the patient to continue scleral lens wear during the day using the same solutions. He also continued to use the lubricant ointment in the evening without a bandage contact lens.
Six months later, he is still successfully wearing his scleral lens. He continues to have some mild blanching nasally and still follows the same wearing schedule.
Case 2:
Move Over, Eye Drops
A 58-year-old Caucasian female presented with a history of dry eye. Her eyes were particularly dry status post blepharoplasty for the upper and lower eyelids OU. She complained of red, burning, tearing and photophobic eyes since her surgery, which may have been a result of over-correction of the upper eyelid; however, no evidence of lagopthalmos was present in either eye. Her ocular history was also significant for posterior subcapsular cataract OD, and she previously wore soft lenses (both daily and two-week replacement). Ocular meds included topical cyclosporine 0.05% one to two times a day and bottled artificial tears one to two times a day; however, she noted no improvement with the eye drops.
• The exam. The patient’s medical history was significant for recurrent herpes simplex virus keratitis. Medications taken were estradiol, progesterone and Valtrex. Visual acuity with glasses was 20/25+1 OD (improved to 20/20+1 with pinhole) and 20/40-2 OS (improved to 20/25+1 with pinhole). Manifest refraction of -10.25+1.00 x 160 OD enabled 20/20-2. Manifest refraction of -8.50+0.75 x 091 in the left eye enabled 20/20-2. Simulated keratometry readings with topography read OD: 42.35 / 065 / 42.24 /155, OS: 42.72 / 098 / 41.82 / 008. Additionally, irregular astigmatism was present OD and regular astigmatism was present OS. Quality of tears was poor and TBUT was two seconds OD.
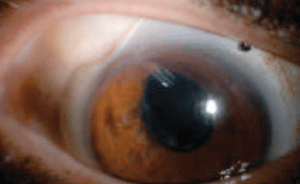
|
|
|
Fig 2. A superotemporal notch was made in this patient’s scleral lens to avoid contact with a Baerveldt glaucoma implant. |
Slit-lamp examination revealed 1+ meibomian gland dysfunction OU. No evidence of lagophthalmos was present in either eye. Conjunctival staining (2+), chemosis (1+) and reduced tear meniscus were also present OU. I observed corneal staining OU; however, the right eye was worse than the left. Tear break-up time (TBUT) was two seconds OD and four seconds OS. IOP was normal in both eyes. I also noted trace nuclear sclerosis OU. The patient’s right eye had a posterior subcapsular cataract. Her optic nerves and maculae were normal OU.
• The fit. To rehabilitate the ocular surface, the initial lenses selected were a 16.5mm diameter lens with a 4.63 sagittal depth.3 I fit the patient with Accu Lens Maxim scleral lenses in Boston XO2 material with a 16.5-mm diameter OU. Lens parameters were: OD 41.00D BC, 16.5mm OAD, 9.50mm OZD, -8.00D power, sag 4.63 and OS 41.00D BC, 16.5mm OAD, 9.5mm OZD, –6.50D power, sag 4.63.
• The result. Visual acuity in each eye was 20/20-1. An over-refraction of +0.25 was present OD; no over-refraction was present OS. Binocular vision without over-refraction was 20/15. Both lenses exhibited good central apical clearance. Less clearance was present superonasally; however, each lens cleared the cornea. The lenses fit well peripherally without blanching and there was no evidence of tear debris or surface debris on either lens.
The patient noticed “tremendous improvement” in her ocular dryness. Thanks to the scleral lenses, she no longer experienced dry eye symptoms while wearing her lenses; without lenses, she still requires artificial tears every 15 minutes during waking hours. She was happy with the vision and comfort the lenses provided. She required no artificial tears while wearing the lenses, but continued to use non-preserved artificial tears and cyclosporine 0.05% BID when not wearing them.
Fortunately, neither mucin debris nor lens surface debris under the lens was present, both of which are common in patients with OSD. Thus, it is important to inform patients of this problem before beginning the fit. Chamber debris is mucous build-up in the reservoir behind lens and tends to be more common in lenses ≥18mm due to a larger fluid reservoir and slower fluid turnover. If mucous debris is present, the lens diameter can be reduced. Patients may also remove the lens and manually clean or rinse and reinsert it one to two times during the day to reduce mucin debris. If possible, reducing lens clearance can reduce mucin debris.
Another tip is to use an artificial tear with increased viscosity (such as Celluvisc, Allergan) with the application of a scleral lens. It is also possible to loosen the peripheral curves to increase fluid exchange or tighten peripheral curves to reduce excessive fluid exchange. If complaints of debris are noted only in the morning, the eye may be soaked with an eye cup before applying lenses, but be sure to convey to patients the importance of disinfecting the cup after use. It is imperative to treat the underlying eyelid disease.
To treat front surface debris, suggest that patients change hand soap to a contact lens or acne treatment hand soap. It is important that hand soap does not contain lotion. Verify that patients are applying make-up after scleral lens insertion. Also, on-eye surface cleaning with a saline-moistened cotton swab or eye shadow applicator is beneficial in removing front surface debris. The lens could also potentially be plasma treated once again. Increased lubrication over the lens throughout the day may also decrease or eliminate debris. Changing to a peroxide-based cleaner and adding enzymatic cleaner is also beneficial. In this case, cyclosporine was used to treat meibomian gland dysfunction.
Case 3:
Time for a Lens Upgrade
A 58-year-old Hispanic female was referred for a contact lens examination. She was experiencing irritated eyes with her current hybrid contact lens and had reverted back to a soft lens for the left eye. She complained of poor vision, especially at distance when driving at night. She also reported double vision when reclining, but not in straight-ahead gaze.
• The exam. The patient’s medical history was significant for diabetes, hypertension, hypothyroidism and sleep apnea. Systemic medications included insulin, metformin, glimepiride, lisinopril, hydrochlorothiazide, levothyroxine and escitalopram. In addition to glaucoma, her ocular history was significant for dry eye OU. Ocular medications were Alphagan (Allergan) and Cosopt (Merck) BID OD. She had a stable IOL in her right eye and a cataract in her left eye. Primary open-angle glaucoma was present in both eyes.
Of note, she had undergone a Baerveldt glaucoma implant six months prior to this examination. Following the glaucoma implant, she developed a right hypertropia and alternating exotropia. This is not uncommon, as persistent restrictive strabismus may occur with glaucoma drainage implants due to scarring between the rectus and oblique muscles.4
Entering vision OD was 20/50+2 without correction and OS was 20/50-2 with a soft contact lens. Anterior segment examination revealed a stable glaucoma drainage device implant located superotemporally in the right eye with a bleb over the plate. The tube was well covered and visible in the anterior chamber. Both eyes exhibited corneal staining (1+ inferior punctate epithelial keratopathy). The posterior chamber IOL was stable OD and mild nuclear and cortical sclerosis was present OS. The patient’s IOPs were 25mm Hg OD and 21mm Hg OS at 1:57pm. Optic nerve examination revealed vertical elongation of the disc with peripapillary atrophy. My-opic degeneration of both the macula and periphery was present OU.
• The fit. My recommended treatment included nonpreserved artificial tears, frequent breaks when reading and using a computer, good water intake and daily omega-3 fatty acid intake. Due to elevated IOP OD, I had her schedule an appointment with the glaucoma surgeon.
I discussed medical management and other options with the patient, and fit her with Maxim (Accu Lens) scleral lenses in Boston XO2 material. Lens parameters were OD 46.00D BC, 15.0mm OAD, 8.00mm OZD, +0.50D power, sag 4.35, 4mm notch (to insert superotemporally) and OS 46.00D BC, 15.4mm OAD, 8.0mm OZD, –13.00D power, sag 4.46 (intermediate/near).
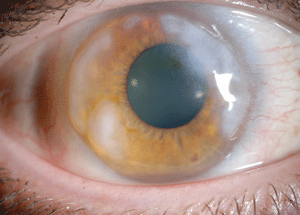
| |
|
Fig 3. Elevated Salzmann’s nodules. |
I targeted the right lens for distance and the left lens for intermediate/near to eliminate diplopia. I also had a superior temporal notch made in the right scleral lens to avoid the Baerveldt glaucoma implant (Figure 2). Putting a notch in a scleral lens may sound complicated, but it is not. First, measure the size (both height and width) of the conjunctival abnormality using a slit beam. Then, measure the height and width of the conjunctival abnormality while the scleral lens is on the eye and mark the scleral lens with a Sharpie or surgical skin marker while its is on the eye. Next, measure the tracing on the lens after removing it from the eye. Finally, call your laboratory consultant to discuss the plan and send the lens to the laboratory.
• The result. Visual acuity at distance was 20/30 OD, 20/30+2 OS and 20/25-2 OU. Additionally, the patient had good computer and near vision, with J1+ OS and J1+ OD at near. She reported incredible comfort with the scleral lenses, both of which fit well with good central apical clearance and good peripheral alignment. I noted no blanching in either eye, and the scleral lens notch was correctly positioned superior temporal in the right eye and did not touch the glaucoma implant. The patient was able to wear the lenses for 15 hours each day. IOPs checked multiple times during a three-month period ranged from 16mm Hg to 18mm Hg in each eye.
Case 4:
Salzmann Who?
A 41-year-old Caucasian female was referred by a corneal specialist for a contact lens fitting. She had a history of Salzmann’s nodular degeneration in both eyes and presented with blurry vision for distance with glasses. She previously tried soft and gas permeable (GP) contact lenses, but vision was not acceptable with soft lenses and she was intolerant to GP lenses. Consequently, she hadn’t worn contact lenses for the last five years. In addition to Salzmann’s nodular degeneration, dry eyes were present OU. Ocular medications included nonpreserved artificial tears as needed, fluorometholone 1% and ketorolac 0.5% daily OU.
• The exam. There was no significant medical history, and the patient was not taking any systemic medications. Visual acuity with glasses was 20/30-2 OD and 20/40 OS. Manifest refraction of -6.75+6.25x123 OD enabled 20/30-2 and manifest refraction of -8.50+6.25 x 059 OS enabled 20/20-2. Simulated keratometry readings with topography read OD: 34.90 / 117 / 25.40 /027 and OS: 42.56 / 066 / 30.74 / 156. Irregular astigmatism was present OU.
Slit lamp examination revealed 1+ meibomian gland dysfunction OU. Mild hyperemia was present OD and the patient’s conjunctiva was white and quiet OS. Scattered elevated Salzmann’s nodules were present from 12 o’clock to 4 o’clock and 7 o’clock to 12 o’clock OD, and elevated Salzmann’s peripheral nodules were also present OS (Figure 3). In addition, I noted an iron line in the mid-periphery OS, but saw no staining in either eye. IOP, optic nerves and maculae were normal OU.
• The fit. To improve vision and rehabilitate the ocular surface, the initial lenses selected were Jupiter GP scleral lenses (Essilor) in Optimum Extra (Contamac) with an 18.2mm diameter. Lens parameters were: OD 44.00D BC, 17.6mm OAD, 9.0mm OZD, -7.75D power and OS 44.25D BC, 18.2mm OAD, 9.0mm OZD, -6.50D power. Visual acuity OD was 20/20-2 and 20/20+2 OS. Good central and peripheral clearance was present in each eye. The lenses cleared the nodules in both eyes.
The patient noticed tremendous improvement with ocular dryness and denied any sensitivity to light. She also reported that her eyes were no longer watery and that vision was improved with very good comfort in each eye. The lenses were stable for three years, after which the right lens began to chafe the cornea superotemporally at 2 o’clock, causing scleral lens intolerance. I referred the patient back to the corneal specialist to consider removal of Salzmann’s nodules in the right eye. Scleral lens wear was discontinued and superficial keratectomy performed at 2 o’clock and 7 o’clock in the right eye.
After four months, she returned for a scleral lens refit. New simulated K readings with Pentacam (Oculus) were OD: 32.20 / 40.1 / 42.20 / 130.1, OS: 27.1 / 138.2 / 46.1 / 48.2. High irregular astigmatism was present OU.
• The refit. The initial lenses selected were Alden Optical’s mini-scleral, fully vaulting lens, Zenlens, available in prolate and oblate designs. I chose an oblate design in Boston XO2 material with a 16.0mm diameter. Lens parameters were: OD 37.5DBC, 16.0mm OAD, 9.0mm OZD, -1.00D power, 4.700 sag and OS 37.5DBC, 16.0mm OAD, 9.0mm OZD, +0.50D power, 4.700 sag. Visual acuity OD was 20/20 and 20/20+1 OS. Good central and peripheral vault was present in each. Most importantly, an even fluorescein pattern was present OU and all peripheral nodules were cleared.
• The result. So far, the patient has been wearing the lenses for four months and reports good vision and comfort with the lenses. She is able to wear them 12 hours per day and uses a +1.25D spectacle prescription over the lenses for near. At the last visit, I ordered a new left lens with the same parameters as above and a +1.75D power for monovision.
Take-Home Tips
With these cases in mind, let's consider what fitting pearls we may glean from the experience. A history of glaucoma surgery—including trabeculectomy, shunt, stent or glaucoma implant—may complicate the fitting of scleral contact lenses because the conjunctiva may be elevated or uneven in the area in which the procedure was performed. Furthermore, excessive pressure or rubbing over tube shunts or valves may compromise IOP and lead to conjunctival and/or tube erosion, which can increase the risk of further complications such as endophthalmitis.
A notch, or an advanced periphery design such as an EyePrintPro prosthetic scleral cover shell (EyePrint Prosthetics) or a PROSE (prosthetic replacement of the ocular surface system) device, can be created in the scleral lens to avoid both pressure on the conjunctiva and contact with the surgical area.
My approach to lens selection depends on multiple factors. For a new fit, I evaluate the corneal abnormality, eye shape, aperture and Pentacam or topography results, then decide on the diameter and lens design. For a refit, I choose a lens that will solve the problem of the current fit. For example, there is less sagittal depth with Maxim lenses than Jupiter lenses. In addition, the Zenlens Oblate design can be used to vault the peripheral cornea. It is also useful for other corneal abnormalities such as post-lasik corneal ectasia.
With sclerals, it is possible to fit inside of conjunctival abnormalities by decreasing the lens diameter or to fit over abnormalities by increasing the lens diameter. Alternatively, it is possible to go around the abnormality by putting a notch in the lens. The notch should never be cut into the optic zone or air will get suck in under the lens.
In cases of glaucoma surgeries or implants, it may be beneficial to avoid the abnormality altogether and to create a notch in the scleral lens. Scleral lens notches are also advantageous when other types of conjunctival abnormality (e.g., an elevated pinguecula or conjunctival cyst) are present.
Finally, when inserting a scleral contact lens, it is important to place it on the eye with the correct orientation. Be sure to inform the staff person who is training the patient on scleral lens application and removal—as well as the patient—about the need for proper lens orientation.
1. Pecego M, et al. Jupiter scleral lenses: the UC Davis Eye Center experience. Eye Contact Lens. 2012 May;38(3):179-82.
2. Visser ES, et al. Modern scleral lenses. Part I: Clinical features. Eye Contact Lens 2007;33(1):13-20.
3. Alipour F, et al. Use of mini scleral contact lenses in moderate to severe dry eye. Cont Lens Anterior Eye. 2012 Dec;35(6):272-6.
4. Schwartz KS, et al. Glaucoma drainage implants: a critical comparison of types. Curr Opin Ophthalmol. 2006 Apr;17(2):181-9.


