Corneal irregularity can be an unfortunate result of corneal disease, trauma or surgery. Depending on severity, an irregular cornea will reduce a patient’s best-corrected visual acuity with glasses or standard soft contact lenses. However, patients are often able to regain functional vision with the use of specialty soft or gas-permeable (GP) designs. Recent advances in instrumentation have significantly improved practitioners’ ability to diagnose, measure and manage patients with corneal irregularity. The four primary categories of advanced technology include: topography, aberrometry, optical coherence tomography and Scheimpflug imaging.
Topography
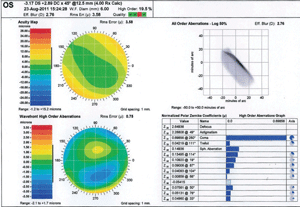
1. Wavescan measurement.
The ability to measure the corneal front surface is an essential component of irregular corneal diagnosis and management. Taking measurements with a keratometer is insufficient, as it is limited to a 3mm to 4mm central zone and curvature measurement of two primary meridians. In addition, the mires of a keratometer are often unreadable as corneal irregularity worsens.
The gold standard for measuring front surface corneal irregularity is videokeratography or corneal topography. Corneal topographers are able to, in some cases, measure in excess of 10,000 data points across the entire corneal surface. A dioptric map of the corneal surface is the primary evaluative tool that a topographer offers the contact lens practitioner. Dioptric values are represented by a relative color-coded scheme. The clinician can use the resultant pattern to interpret, diagnose and classify the corneal irregularity.
There are two basic types of dioptric maps: sagittal and tangential. Sagittal maps, also called axial maps, are set up with algorithms that assume the cornea is spherical, which makes them less accurate for peripheral corneal evaluation and measurement. Tangential maps are based upon instant radius of curvature, making them inherently more accurate for diagnostic and lens fitting purposes.1
Corneal classification based upon topography allows the practitioner to more efficiently fit an eye with irregularity because the shape of the cornea plays a critical role in determining the type of design that will work best for the patient. For example, a patient with an oblate cornea will probably do best with a reverse geometry design or a patient with mild keratoconus may do best with an aspheric back surface corneal GP design. Practitioners often use corneal topographic data to help predict the first diagnostic lens to trial after choosing a specific lens design. It is customary for a manufacturer’s fitting guide to recommend an initial diagnostic lens based upon a formula that uses simulated keratometry measurements taken from corneal topography.
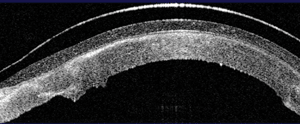
2. OCT image of a scleral lens vaulting the corneal surface.
In a 2010 study, Luigina Sorbara, O.D., M.Sc., showed improved success by using tangential topographic data in determining the back optic zone diameter in tandem with the overall diameter when fitting corneal GP lenses for keratoconus.2 However, keep in mind that many specialty lens designs are based upon sagittal depth of the anterior ocular surface (cornea and sclera) and dioptric topographical results are probably not much help when choosing the first diagnostic lens. For example, in another 2010 survey, Graeme Young, B.Sc., M.Phil., and colleagues, found that although placido-based topography was a better predictor of base curve for soft lens fitting compared to keratometry, topography still wasn’t reliable enough for accurate base curve selection.3 Muriel M. Shornack, O.D., and Sanjay V. Patel, M.D., found only a weak predictive relationship between base curve selection for a scleral lens design and topography.4 So, although topographical dioptric results are essential for diagnosing and classifying the type of corneal irregularity, they may not be always useful in base curve selection for diagnostic lens fitting.
Some topographers are able to produce an elevation map of the cornea based on a reference sphere, which helps to predict areas of potential clearance or bearing for corneal GP lenses. In addition, many topographers have software that will allow the clinician to virtually fit a cornea GP lens based upon topographical results. Empirically fitting GP lenses this way has the potential to dramatically improve fitting efficiency. With this technology, the practitioner can virtually alter design parameters and observe how changes might affect the lens fit.

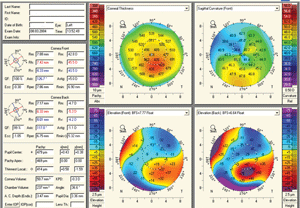
3. Oculus Pentacam.
Christine Sindt, O.D., and colleagues conducted a study to evaluate virtual fittings of keratoconus.5 The aim of the study was to determine how close virtually produced fluorescein patterns correlated with actual fluorescein patterns in 31 keratoconous patients who were fit using a bi-aspheric keratoconic design. They found that 74% of eyes showed a good match between theoretical and actual fluorescein patterns.5 Ultimately, the success of these results hinged on the ability of the topographer to produce a quality ring reflection from the placido disc. In fact, when looking at the cases where a quality ring image was produced, there was a 95% accuracy between the virtual prediction and the actual results.5
Keratoconic severity, corneal scarring and tear break-up can all contribute to poor ring quality. It’s also important to remember that virtual fitting patterns do not account for lid interaction, which can significantly affect the fitting relationship of a corneal GP lens.
Aberrometry
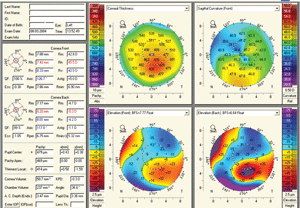
4. Pentacam readout of a keratoconus patient.
Irregular corneal astigmatism induces higher aberrations to the visual system that are not corrected for by glasses or standard contact lenses. Depending on severity, these aberrations can lead to significant visual disability. With modern instrumentation, we can quantify aberrations that fall outside the lower bounds—such as defocus and astigmatism—that practitioners routinely measure.
One of the primary methods of measuring higher-order aberrations involves Shack-Hartmann principles, which produces a measurable wavefront. For an emmetropic eye without any aberrations, the resulting wavefront of light will be flat.6 Any lower- or higher-order aberration will cause the wavefront to distort, which can then be represented mathematically as root mean square (RMS), color-coded map or point spread function (figure 1).
Each specific type of aberration can be mathematically represented by what is known as a Zernike polynomial. The common higher-order Zernike polynomials have names such as spherical, coma and trefoil. Typically, contact lens practitioners rely on GP or specialty soft contact lenses that are able to mask higher-order aberrations caused by front surface corneal irregularity.
Although most irregular corneal patients are able to achieve functional vision with these specialty lens designs, many of them will still have less than perfect visual acuity. Some patients, especially those with keratoconus, may complain of ghosting, halos and/or glare resulting from uncorrected back surface corneal irregularity that is not corrected for by their specialty lens.7,8
Current technology can produce custom contact lenses that incorporate residual higher aberration control for individual patients. In 2007, Jason D. Marsack, M.S., and colleagues reported on a case of a habitual soft lens-wearing patient with moderate keratoconus who was fit with wavefront-guided soft contact lenses.9 The patient showed 1.5 lines of visual acuity improvement and a 50% reduction in higher-order aberrations. Costas F. Katsoulos and colleagues showed improvement in visual performance for two keratoconic patients who wore customized hydrogel contact lenses implementing correction for vertical coma.10
Although the technology to measure and implement higher-order aberration correction into contact lenses is currently available, there are three hurdles that must be accounted for in order to maximize success.
• A successfully fit GP or soft contact lens is going to have on-eye translation and rotation that can induce higher aberrations. For example, a decentered lens that incorporates aspheric optics to control for spherical aberration can induce coma, which can nullify expected improvement.
John De Brabander, Ph.D., and colleagues advise that translation errors should not exceed 0.5mm, after reporting translation errors significantly affected performance on a simulated optical performance of custom wavefront soft contact lenses for keratoconus.11 Because it is expected that contact lenses will experience these translations and rotations, Antonio Guirao, Ph.D., and colleagues suggest partial correction of every aberration term in order to minimize induced wavefront aberrations.12
• Not all aberrations affect the eye equally. For example, coma negatively affects vision by a factor of two as compared to spherical aberration.6 Some Zernike modes may interact when combined to improve visual acuity despite increasing the total wavefront error.13,14 Therefore, trying to completely reduce one or all aberrations may not maximize the patient’s success.
• Patients may build up tolerance. Patients who have a longstanding history of uncorrected higher-order aberrations may have neurally adapted to their visual blur and may not be able to immediately appreciate the improved optics.15,16
Optical Coherence Tomography
5. Bellin ambrosion BAD II ectasia.
Optical coherence tomography (OCT) is able to produce two-dimensional cross-sectional images of the anterior ocular surface. These high-resolution images allow for detailed analysis of the corneal layers. Diagnostically, this provides magnified visualization and biometric measurement. The OCT instruments being developed today have the ability to perform both anterior and posterior segment measures, which means that practitioners only need to purchase one instrument to gain two highly useful technologies. Examples of these combination systems include the Cirrus (Carl Zeiss Meditec), the RTVue (Optovue) and the 3D OCT (Topcon). Anterior segment OCT is still pending FDA approval.
OCT imaging of the cornea and sclera has the potential to improve the evaluation and design of contact lenses (figure 2).17,18 Greg Gemoules, O.D., reported on nine patients that were successfully fit with scleral GP lenses utilizing sagittal depth and chord measurements obtained taken from a Visante OCT (Carl Zeiss Meditec).18
For the ICD scleral lens design, the practitioner can use either sagittal depth measurement from an OCT or weighted height measurement from a Medmont topographer to choose the initial diagnostic lens. The future of this technology will someday allow for a single measurement that will profile the anterior segment, so that an empirical soft or scleral lens can be customized directly from the collected data.
Scheimpflug Imaging
Tomography is the creation of a 2-D image from a slice or section through a 3-D object. Certain instruments utilize a specific technique of imaging called Scheimpflug imaging, which has the advantage of extending the depth of focus—a 2-D image capture can create a 3-D construct. The most widely used example of such technology is the Pentacam (Oculus); other devices include the Galilei (Ziemer) (figure 3).
This technology is quite diverse in its clinical applications. Advantages include the ability to collect 360º imaging of the anterior segment, both anterior and posterior corneal elevation-based topography, global corneal pachymetry, anterior chamber analysis, anterior segment optical densitometry, IOL planning and contact lens design based on anterior segment tomography. As such, the applications are far reaching into areas of corneal analysis, cataract management, glaucoma analysis and IOL planning.
The most widely used application of Scheimpflug technology is the ability to detect corneal ectasia (figure 4). Due to the measurement of posterior corneal elevation and global pachymetry, abnormalities associated with ectasia are exquisitely detected, quantified and monitored. Specific software programs such as the Belin/Ambrosio II on Pentacam have the ability to clearly differentiate normal from ectatic corneas, thus allowing for earlier detection of ectasia and avoidance of performing keratorefractive procedures on patients who may be poor candidates (figure 5). Belin has recently utilized Pentacam to differentiate pellucid marginal degeneration from keratoconus with a pseudo-PMD anterior corneal topography through the analysis of global pachymetry.19
Advances in instrumentation have significantly improved the diagnostic and fitting success for irregular corneal management. Practitioners can utilize topography-based software that enables virtual contact lens fitting. Ultimately this can improve efficiency, especially for the novice contact lens practitioner.
Scheimpflug tomography allows for comprehensive corneal and anterior segment analysis with unique evaluation of the posterior corneal surface and global pachymetry, while aberrometry can quantify corneal irregularity. These measurements can then be used to add higher-order aberration control to contact lenses to improve visual outcomes. Ocular coherence tomography will improve design and fitting success for soft and scleral type lenses used for difficult cases.
Dr. DeNaeyer is the clinical director for Arena Eye Surgeons in Columbus, Ohio. His primary interests include specialty contact lenses. He is also a consultant and advisor to Visionary Optics, Bausch + Lomb and Aciont.
Dr. Eiden is the president and medical director of North Suburban Vision Consultants, Ltd., co-founder and president of EyeVis Eye and Vision Research Institute and teaches at the University of Illinois at Chicago Medical Center and Indiana, Illinois, Salus and UMSL colleges of optometry. Dr. Eiden is a consultant for Oculus.
1. Rabinowitz YS. Tangential vs sagittal videokeratographs in the “early” detection of keratoconus. Am J Ophthalmol. 1996 Dec;122(6):887-9.
2. Sorbara L, Dalton K. The use of video-keratoscopy in predicting contact lens parameters for keratoconic fitting. Cont Lens Anterior Eye. 2010 Jun;33(3):112-8.
3. Young G, Schnider C, Hunt C, Efron S. Corneal topography and soft contact lens fit. Optom Vis Sci. 2010 May;87(5):358-66.
4. Schornack MM, Patel SV. Relationship between corneal topographic indices and scleral lens base curve. Eye Contact Lens. 2010 Nov;36(6):330-3.
5. Sindt CW, Grout TK, Kojima R. Evaluating virtual fitting for keratoconus. CL Spectrum. 2011 May. Available at:
www.clspectrum.com/articleviewer.aspx?articleid=105575 (accessed March 2012).
6. Kolbaum PS, Bradley A. Correcting aberrations with contact lenses. CL Spectrum. 2007 Nov. 7. Nakagawa T, Maeda N, Kosaki R, et al. Higher-order aberrations due to the posterior. Invest Ophthalmol. 2009 Jun;50(6):2660-5.
8. Negishi K, Kumanomido T, Utsumi Y, Tsubota K. Effect of higher-order aberrations on visual function in keratoconic eyes with a rigid gas permeable contact lens. Am J Ophthalmol. 2007 Dec;144(6):924-9.
9. Marsack JD, Parker KE, Niu Y, et al. On-eye performance of custom wavefront-guided soft contact lenses in a habitual soft lens-wearing keratoconic patient. J Refract Surg. 2007 Nov;23(9):960-4.
10. Katsoulos C, Karageorgiadis L, Vasileiou N, et al. Customized hydrogel contact lenses for keratoconus incorporating correction for vertical coma aberration. Ophthalmic Physiol Opt. 2009 May;29(3):321-9.
11. de Brabander J, Chateau N, Marin G, et al. Simulated optical performance of custom wavefront soft contact lenses for keratoconus. Optom Vis Sci. 2003 Sep;80(9):637-43.
12. Guirao A, Cox IG, Williams DR. Method for optimizing the correction of the eye’s higher-order aberrations in the presence of decentrations. J Opt Soc Am A Opt Image Sci Vis. 2002 Jan;19(1):126-8.
13. Applegate RA, Marsack JD, Ramos R, Sarver EJ. Interaction between aberrations to improve or reduce visual performance. J Cataract Refract Surg. 2003 Aug;29(8):1487-95.
14. Applegate, RA, Sarver EJ, Khemsara V. Are all aberrations equal? J Refract Surg. 2002 Sep-Oct;18(15):S556-62.
15. Sabesan R, Yoon G. Neural compensation for long-term asymmetric optical blur to improve visual performance in keratoconic eyes. Invest Ophthalmol Vis Sci. 2010 Jul 51(7):3835-9.
16. Sabesan R, Yoon G. Visual performance after correcting higher order aberrations in keratoconic eyes. J Vis. 2009 May 13;9(5):6.1-10.
17. Hall LA, Young G, Wolffsohn JS, Riley C. The influence of corneoscleral topography on soft contact lens fit. Invest Ophthalmol Vis Sci. 2011 Aug 29;52(9):6801-6.
18. Gemoules G. A novel method of fitting scleral lenses using high resolution optical coherence tomography. Eye Contact Lens. 2008 Mar;34(2):80-3.
19. Belin MW, Asota IM, Ambrosio R, et al. What’s in a name: keratoconus, pellucid marginal degeneration and related thinning disorders. Am J Ophthalmol. 2011 Aug;152(2):157-62.


