The conjunctiva—the thin, translucent membrane that lines the inside of the eyelids (palpebral conjunctiva) and covers the sclera (bulbar conjunctiva)—is thinnest along the eyelid margin and thickest in the fornices. It is composed of two layers: a stratified, non-keratinized epithelial layer consisting mostly of goblet cells and a submucosa layer containing mostly antimicrobial and inflammatory response cells, such as macrophages and mast cells.1 Its main functions include protecting the soft tissues of the eyelid and orbit, allowing extensive movement of the eye without damaging soft tissue, serving as a source of antimicrobial and other immunological agents and producing the mucin layer of the tear film.
Soft contact lens wear causes the conjunctiva to respond in various ways. The association between conjunctival changes and symptomotology is important to consider, as it can play a role in the outcome of a fitting. However, it is not always entirely clear whether these physiological changes are the underlying cause of contact lens discomfort. This article describes several conjunctival findings that are important to consider in soft contact lens wearers.
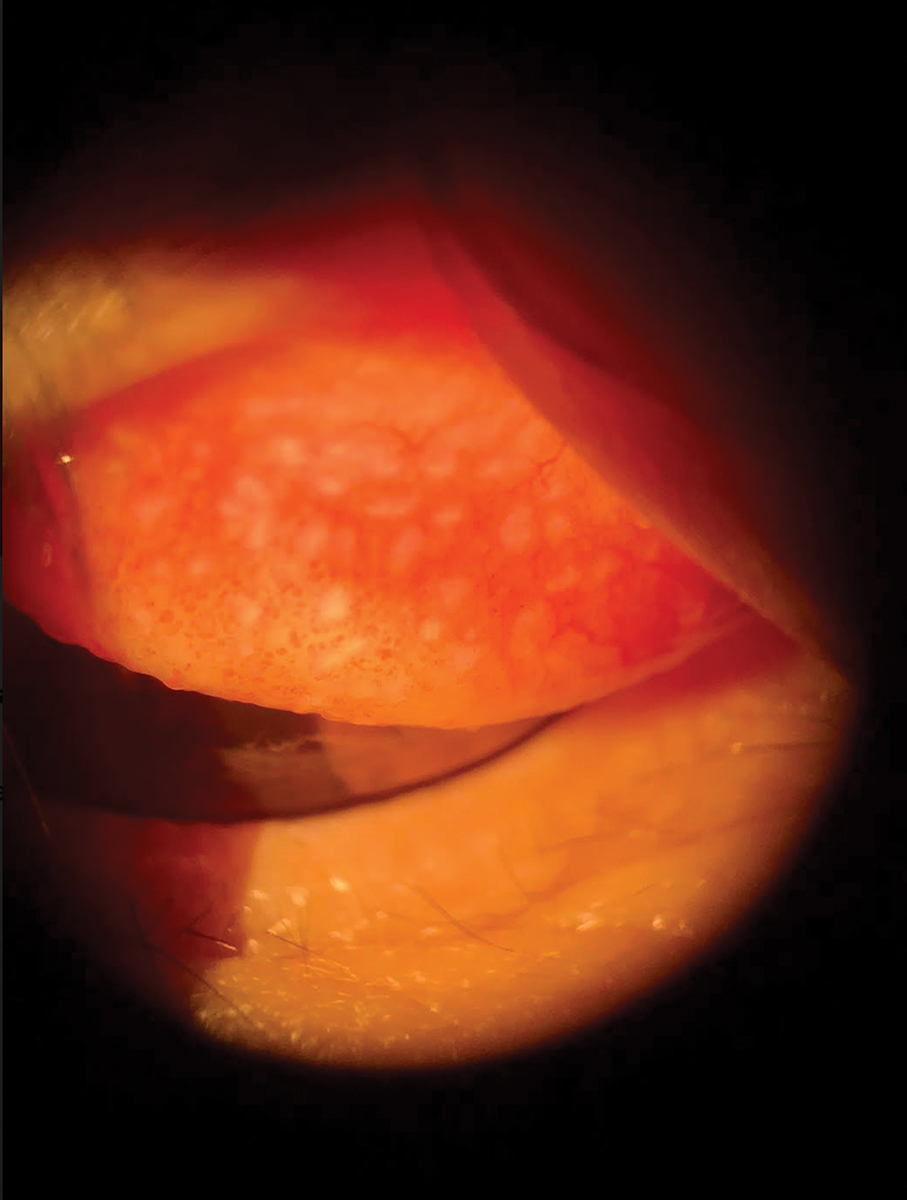 |
Temporarily discontinuing lens wear can help manage GPC. Click image to enlarge. |
1. Giant Papillary Conjunctivitis (GPC)
This is a non-infectious inflammatory response of the superior tarsal palpebral conjunctiva due to mechanical irritation from chronic eyelid movement over a foreign object. Most commonly, GPC is associated with contact lens wear; however, similar reactions have been noted with exposed ocular sutures, filtering blebs, ocular prosthesis, scleral buckles and elevated corneal scars.2
Pathophysiology. GPC results in papillary changes in the palpebral conjunctiva as part of an immunoglobulin E–mediated hypersensitivity reaction to the presence of a foreign object.2 Eyelid movement can stimulate the resulting inflammatory response, especially as people blink tens of thousands of times per day.3 With age, this rate increases even more.3 The papillary inflammation causes papillae to grow in size as GPC advances. Large papillae (greater than 0.3mm in diameter) on the tarsal conjunctiva are a classic sign of the disease.4
The polymer of a contact lens can also influence GPC development. The type of polymer can impact the amount of deposits that form on the surface of a lens. For example, polymers that have higher water content and ionic properties attract larger amounts of protein deposits compared with lenses with lower water content.5,6 The push toward increased oxygen permeability with silicone hydrogel contact lenses unfortunately makes the lenses more susceptible to protein deposits. In addition, the higher modulus of silicone hydrogel lenses makes them stiffer, which can cause even more mechanical trauma.7
Management. Since the pathophysiology of GPC involves immune and mechanical components, treating both is important. GPC is manageable with nontherapeutic or therapeutic methods. Nontherapeutic methods that can effectively prevent and treat GPC include discontinuing lens wear temporarily or permanently, changing to a daily disposable—or at least a more frequent replacement cycle to accommodate lens parameters that aren’t offered in a daily disposable option—or gas permeable lens, switching to a preservative-free disinfectant solution and using cold compresses or lubricating eye drops. Taking into account contact lens edge thickness and design are other strategies to prevent recurrence.
One therapeutic option that can be helpful in treating the inflammation associated with GPC is topical steroids. Although steroids can provide prompt symptom relief, they can cause potential complications, such as healing impairment and infection risk, and side effects, including cataracts, glaucoma and increased intraocular pressure. Another option is antihistamines or mast cell stabilizers. Although GPC is not primarily a mast cell–mediated reaction, these alternatives may allow us to get ahead of the disease before it progresses.
Proactively trying to understand GPC could ultimately help prevent it and promote a healthy interaction between the conjunctiva and the ocular surface.
2. Pyogenic Granuloma (PG)
These benign vascular proliferations can occur on the skin and mucous membranes, including the conjunctiva.8 They appear as small or large and smooth or lobulated vascular nodules that can grow rapidly. Symptoms include irritation, foreign body sensation and bleeding.8 PGs can occur in all age groups and appear to affect both men and women equally.
Pathophysiology. PGs are presumed to represent an abnormal reaction to healing, most commonly occurring in sites of injury that involve chronic chalazia or surgery.9 However, the true etiology remains unknown. Histological slides reveal a mixture of inflammatory cells, blood vessels and connective tissue.9 Inflammatory cells include lymphocytes, plasma cells and scattered neutrophils. Newly exhibited blood vessels are immature.
Vascular proliferation occurs in three stages: cellular phase, capillary or vascular phase and involutionary phase.8 Early lesions contain numerous capillaries and venules with prominent endothelial cells arrayed radially toward the epithelial surface. Mature lesions exhibit a fibromyxoid stroma that separates the lesion into lobules. A major driver in the pathogenesis of PG appears to be a mutation within the endothelial cells.8
Management. A first-line therapeutic treatment for PG is ophthalmic drops. Since the pathophysiology of PG is inflammatory, treatment with topical corticosteroids is effective in controlling and reducing the size of the lesion. For those who do not respond to topical agents, surgical excision or cryotherapy is advised.
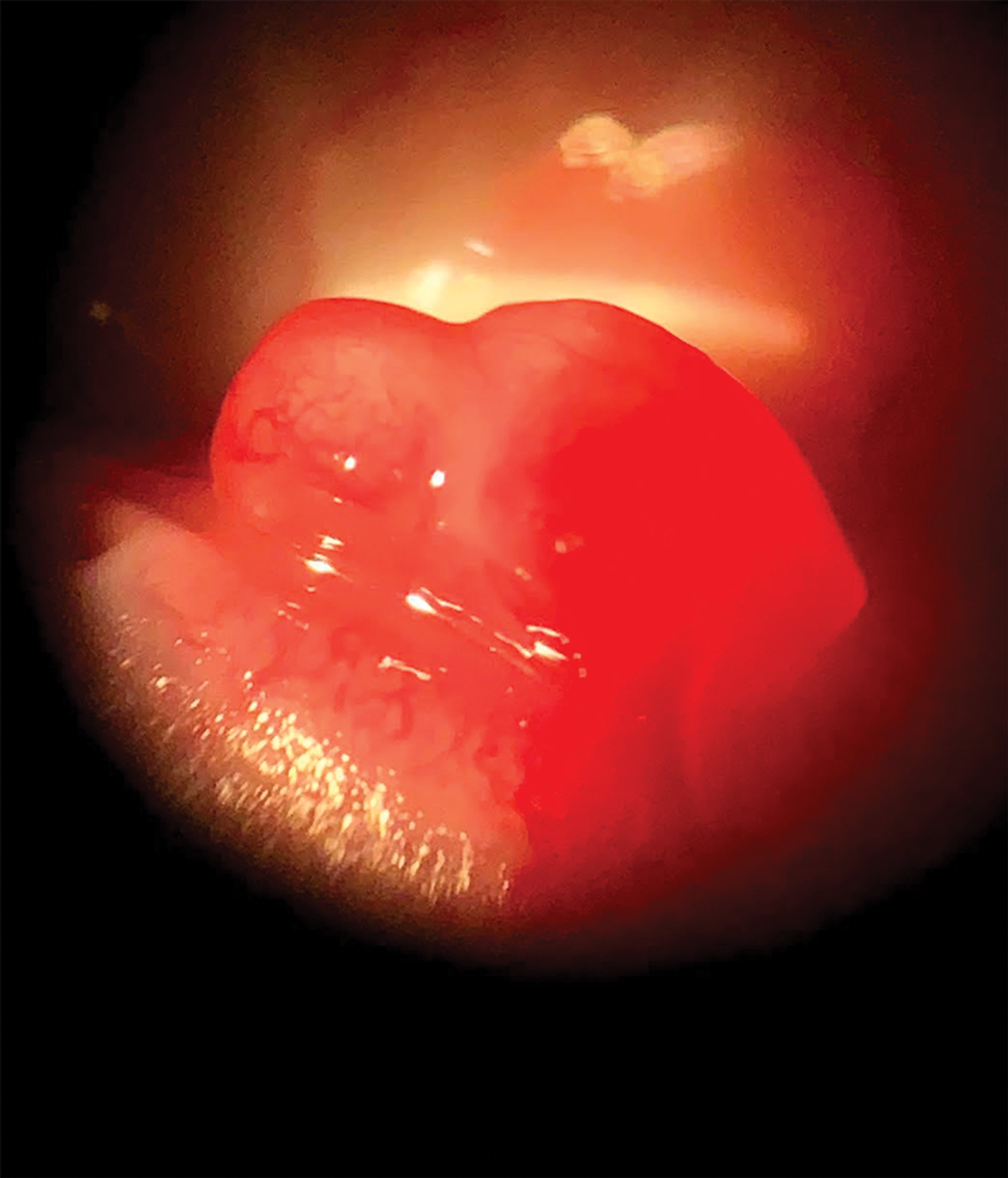 |
| A pyogenic granuloma can appear small or large and smooth or lobular. Click image to enlarge. |
3. Conjunctivochalasis
This is defined as a loose, redundant conjunctiva. As we age, our body’s tissues, including the ocular surface, lose their elasticity. A common sign the ocular surface is experiencing this is chalasis. Often located in the inferior-temporal conjunctiva, chalasis tends to increase in incidence and magnitude with age.10,11 Many symptoms associated with chalasis are similar to those of dry eye disease and could include eye pain, blurred vision, epiphora, discomfort and dryness.12
Pathophysiology. While the true cause of chalasis is not yet known, it is hypothesized that the etiology is multifactorial.13 It may result from local trauma, age-related connective tissue degradation, inflammation or delayed tear clearance.13,14 The dominant theory was derived from the thought that chalasis is the result of an age-related degradation of conjunctival elastic fibers from repeated mechanical insult of the eyelids on the conjunctiva.15 This may escalate with contact lens use, seeing how contact lens wearers, especially gas permeable users, are more likely to have conjunctivochalasis.16 This risk increases with years of wear, as mechanical insult causes the elastic fibers to degrade over time and creates redundant tissue.15,16
The mechanical trauma is also thought to activate an inflammatory cascade that breaks down the conjunctival connective tissue, which may lead to chalasis.17 Another study proposed the idea that chronic inflammation from decreased tear clearance allows inflammatory or degradation mediators to build up on the ocular surface and break down the conjunctival fibers over time, creating redundant tissue.18 It showed that stress on the ocular surface from ultraviolet radiation, oxidative stress, dry eyes and mechanical trauma could lead to an increased production of inflammatory molecules.18
The increase in inflammatory molecules due to insult can activate matrix metalloproteinases (MMPs).18,19 The decreased tear clearance encourages MMPs to remain on the ocular surface for even longer, allowing for compounding conjunctival damage that leads to more redundant tissue. This creates a continuous cycle of worse tear flow, more redundant tissue and possible punctum blockage to keep more toxic tears on the conjunctiva for longer periods of time.
Management. Treatment of chalasis varies depending on the severity of each case. Generally, no treatment is needed for asymptomatic patients. Topical pharmaceutical intervention can help address inflammation and stabilize the tear film in symptomatic patients. Soft corticosteroids can target inflammation but may require extended periods of use. In addition, antihistamines and mast cell stabilizers can assist in managing any concurrent allergic-like conjunctivitis. Lubricants, such as artificial tears and gels, can help stabilize the tear film. If discomfort continues to persist despite maximum therapy, consider conjunctivoplasty.
4. Lid Wiper Epitheliopathy (LWE)
This refers to an epithelial disturbance of the marginal conjunctiva of the upper and lower eyelids. It is an epitheliopathy of the squamous epithelium of the conjunctiva. The lid wiper region is the portion of the marginal conjunctiva of the upper and lower eyelid that spreads the tear film over the ocular surface.20 It is located in the area of the eyelid that rubs against the ocular surface, posterior to the line of Marx (the mucocutaneous junction between the palpebral conjunctiva and the eyelid positioned posterior to the meibomian glands).
Pathophysiology. LWE is thought to be the result of increased mechanical friction between the lid wiper region and the ocular surface that leads to epithelial compromise and inflammation.21 This increase in friction could be due to inadequate lubrication, contact lens wear or environmental factors. You can observe the disturbance to the conjunctival epithelium of the lid wiper region through vital staining of the upper and lower lid margins.
LWE is more prevalent in contact lens wearers and has been observed in both gas permeable and soft lens users.22 One study found LWE in over 80% of contact lens participants.23 It can be seen in both symptomatic and asymptomatic patients.20 Histological studies demonstrated that the goblet cells in the lid wiper epithelium produce gel-forming mucins, which create a thin mucin-water gel that lubricates the surfaces of the lid wiper region and the ocular surface.24 The thin gel protects the lid wiper region from damage by facilitating low friction during blinking. Contact lenses can separate the thin gel, causing inadequate lubrication.
A study found that the microvascular response of the lid wiper region was significantly correlated with contact lens discomfort, suggesting that friction could be related to both the hyperemic response and lid wiper staining.25 Another team of researchers observed an upregulation of Langerhans cells (LCs) in the lid wiper region during contact lens discomfort, indicating that LWE may have an inflammatory component.26 LCs act as antigens within the squamous epithelium of the epidermis and help lymphocytes recognize and react to an immune response.27
Management. Adequately lubricating the ocular surface is essential to minimize friction and manage LWE. Rewetting drops containing carboxymethylcellulose and hyaluronic acid can improve comfort and LWE staining.28 Metastable lipid emulsion drops are also effective in diminishing the severity of LWE and any associated symptoms.29 Other options include using punctal plugs, applying ointment at night and decreasing the modulus of the contact lens.
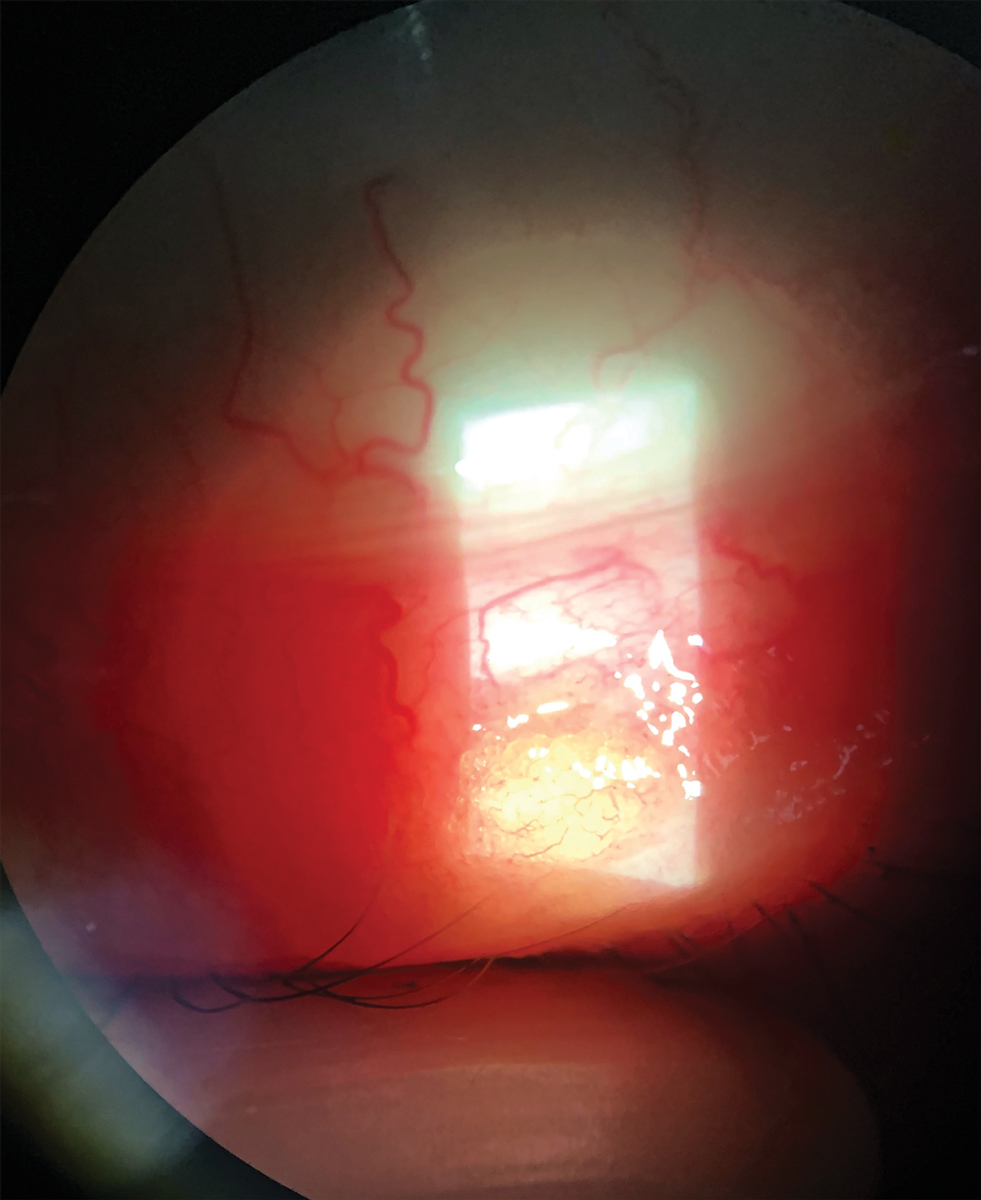 |
| Treat chalazia conservatively by employing noninvasive methods. They should resolve within a month. Click image to enlarge. |
5. Chalazion
This benign inflammatory condition of the eyelid starts as a tender swelling of the upper or lower eyelid. While styes are caused by an infected hair follicle along the lid margin, chalazia are the result of blockage and inflammation of the oil-secreting glands of the eyelid.30 They are common, but their exact incidence is unknown.30 They occur more commonly in adulthood and affect males and females equally. Patients with underlying conditions, such as rosacea, seborrheic dermatitis and blepharitis, are more prone to multiple and recurrent chalazia.30
Pathophysiology. Blockages in the eyelid glands that secrete oil create lipid inspissation in the meibomian gland that can lead to the rupture and release of lipids into the surrounding tissues.31 This results in a granulomatous inflammatory reaction. A study looking into the cytopathology of chalazia revealed that this condition may involve two patterns of granulomatous inflammation.32 A chalazion may either be a mixed-cell or a suppurating granuloma.32 These two patterns of granulomatous inflammation reflect the spectrum of changes in the course of the condition. The inflammatory response from lipid inspissation can create a continuous cycle that causes the chalazion to enlarge and break through the tarsal plate.
Management. It is common practice to treat chalazia conservatively. Employ noninvasive methods, such as lid scrubs and hot compresses with or without a digital massage. The majority of chalazia clear up within one month of these conservative measures.30 Although antibiotics are generally not indicated for chalazia, consider a short course of systemic therapy for lesions with associated blepharitis. Doxycycline is the drug of choice because of its dual antimicrobial and anti-inflammatory properties, but azithromycin can be effective as well.33 In patients who don’t respond to conservative therapy, intralesional steroid injection has long been an effective option because the inflammatory cells comprising chalazia are sensitive to steroids. Alternatively, surgical incision and drainage may be necessary.
Most of the focus remains on the cornea and ocular surface when it comes to contact lenses; however, it is important not to overlook the conjunctiva. Contact lenses interact with both the bulbar and palpebral conjunctival regions and, thus, they can have adverse effects on a contact lens wearer. There are multiple conjunctival considerations to take into account with contact lens wear. Contact lens users can present with conditions that are multifactorial, so understanding conjunctival comorbidities is of extreme importance. Now that we have a better understanding of these conditions, we can use our knowledge in clinical practice to more effectively diagnose and treat them in our patients.
Dr. Asnaashari graduated from the UC Berkeley School of Optometry in 2018 and is a therapeutic- and glaucoma-certified optometrist in Sacramento, CA. One of his areas of expertise is specialty contact lenses.
1. Remington LA, Goodwin D. Clinical Anatomy and Physiological of the Visual System, 3rd Ed. Boston: Butterworth-Heinemann; 1988. 2. Barnett M. Rethinking GPC: a new look at an old problem. Rev Cornea Cont Lens. 2015;152(2):10-3. 3. Sforza C, Rango M, Galante D, et al. Spontaneous blinking in healthy persons: an optoelectronic study of eyelid motion. Ophthalmic Physiol Opt. 2008;28(4):345-53. 4. Allansmith MR, Korb DR, Greiner JV, et al. Giant papillary conjunctivitis in contact lens wearers. Am J Ophthalmol. 1977;83(5):697-708. 5. Fowler SA, Korb DR, Allansmith MR. Deposits on soft contact lenses of various water contents. CLAO J. 1985;11(2):124-7. 6. Minno GE, Eckel L, Groemminger S, et al. Quantitative analysis of protein deposits on hydrophilic soft contact lenses: I. Comparison to visual methods of analysis. II. Deposit variation among FDA lens materials groups. Optom Vis Sci. 1991;68(11):865-72. 7. Kim E, Saha M, Ehrmann K. Mechanical properties of contact lens materials. Eye Contact Lens. 2018;44(Suppl 2):S148-56. 8. Efron N. Contact Lens Complications, 4th Ed. Philadelphia: Elsevier; 2019. 9. Norn MS. Fluorescein vital staining of the cornea and conjunctiva. Acta Ophthalmol (Copenh). 1964;42:1038-45. 10. Gumus K, Pflugfelder SC. Increasing prevalence and severity of conjunctivochalasis with aging detected by anterior segment optical coherence tomography. Am J Ophthalmol 2013;155(2):238-42. 11. Balci O. Clinical characteristics of patients with conjunctivochalasis. Clin Ophthalmol. 2014;8:1655-60. 12. Bert BB. How to manage conjunctivochalasis. Rev Ophthalmol. September 12, 2017. [Epub ahead of print]. 13. Lozano AFI, Larrazabal LI, Nallasamy N, et al. Conjunctivochalasis. American Academy of Ophthalmology. eyewiki.aao.org/conjunctivochalasis. October 13, 2019. Accessed March 26, 2020. 14. Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol. 1998;43(3):225-32. 15. Watanabe A, Yokoi N, Kinoshita S, et al. Clinicopathologic study of conjunctivochalasis. Cornea. 2004;23(3):294-8. 16. Mimura T, Usui T, Yamamoto H, et al. Conjunctivochalasis and contact lenses. Am J Ophthalmol. 2009;148(1):20-5.e1. 17. Huang Y, Sheha H, Tseng S. Conjunctivochalasis interferes with tear flow from fornix to tear meniscus. Ophthalmology. 2013;120(8):1681-7. 18. Meller D, Li DQ, Tseng SC. Regulation of collagenase, stromelysin, and gelatinase B in human conjunctival and conjunctivochalasis fibroblasts by interleukin-1beta and tumor necrosis factor-alpha. Invest Ophthalmol Vis Sci. 2000;41(10):2922-9. 19. Erdogan-Poyraz C, Mocan MC, Bozkurt B, et al. Elevated tear interleukin-6 and interleukin-8 levels in patients with conjunctivochalasis. Cornea. 2009;28(2):189-93. 20. Srinivasan S. Lids, friction and contact lens wear. Rev Cornea Cont Lens. 2016;153(7):20-2. 21. Efron N, Brennan NA, Morgan PB, et al. Lid wiper epitheliopathy. Prog Retin Eye Res. 2016;53:140-74. 22. Shiraishi A, Yamaguchi M, Ohashi Y. Prevalence of upper- and lower-lid-wiper epitheliopathy in contact lens wearers and non-wearers. Eye Cont Lens. 2014;40(4):220-4. 23. Schulze MM, Srinivasan S, Hickson-Curran SB, et al. Lid wiper epitheliopathy in soft contact lens wearers. Optom Vis Sci. 2016;93(8):943-54. 24. Knop N, Korb DR, Blackie CA, et al. The lid wiper contains goblet cells and goblet cell crypts for ocular surface lubrication during the blink. Cornea. 2012;31(6):668-79. 25. Deng Z, Wang J, Jiang H, et al. Lid wiper microvascular responses as an indicator of contact lens discomfort. Am J Ophthalmol. 2016;170:197-205. 26. Alzahrani Y, Colorado L, Pritchard N, et al. Inflammatory cell upregulation of the lid wiper in contact lens dry eye. Optom Vis Sci. 2016;93(8):917-24. 27. Jaitley S, Saraswathi TR. Pathophysiology of Langerhans cells. J Oral Maxillofac Pathol. 2012;16(2):239-44. 28. Nichols JJ, Lievens CW, Bloomenstein MR, et al. Dual-polymer drops, contact lens comfort, and lid wiper epitheliopathy. Optom Vis Sci. 2016;93(8):979-86. 29. Herman JP, Korb DR, Greiner JV, et al. Treatment of lid wiper epitheliopathy with a metastable lipid emulsion or a corticosteroid. Invest Ophthalmol Vis Sci. 2005;46(13). 30. Jordan GA, Beier K. Chalazion. StatPearls. www.ncbi.nlm.nih.gov/books/NBK499889/. February 11, 2020. Accessed March 26, 2020. 31. Gilchrist H, Lee G. Management of chalazia in general practice. Aust Fam Physician. 2009;38(5):311-4. 32. Dhaliwal U, Arora VK, Singh N, et al. Cytopathology of chalazia. Diagn Cytopathol, 2004;31(2):118-22. 33. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease, 7th Ed. Philadelphia: Wolters Kluwer; 2017. |


