Presbyopia is the only ocular condition with a prevalence of 100% in patients older than 50.1 While not all presbyopes require correction, due to congenital monovision, it’s important to realize that accommodation declines steadily with age for everyone.
To many, presbyopia may seem like it’s just another annoyance that comes with aging. Uncorrected presbyopia, however, can result in severe visual impairment and deprive someone of a satisfactory quality of life and opportunities requiring working near vision. The global burden of uncorrected presbyopia in terms of productivity loss is estimated to be just over $11 billion annually.2
Luckily, the condition is correctable. Those who are motivated to shed their spectacles can pursue contact lenses. Some go a step further and seek complete visual independence, and many surgical options are available. This article discusses current and future therapies available to the presbyopic population beyond spectacles and contact lenses.
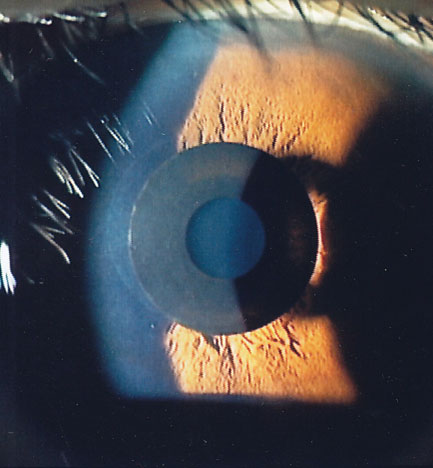 |
| Fig. 1. The Kamra is designed to increase depth of focus. Photo: Minoru Tomita, MD. Click image to enlarge. |
Surgical Therapies
Device companies have come up with three basic surgical strategies for providing permanent, or at least long-term, correction of near vision loss in presbyopes: (1) making changes directly within the optical pathway, (2) altering the underlying architecture and function of the accommodative mechanism outside the optical pathway and (3) inducing changes within the lens itself.
Kamra (AcuFocus). Launched in 2015, this is the only FDA-approved synthetic corneal presbyopic implant.3 It consists of a 6.0µm-thick laser-fenestrated disc of polyvinylidene fluoride that is 3.8mm in diameter with a 1.6mm central aperture.3 The device is positioned over the pupillary axis inside a femtosecond laser-created pocket at a corneal depth of 40% to achieve near monovision.3 The Kamra’s small aperture extends the eye’s depth of focus (DOF), providing uncorrected near visual acuity (UCNVA) of about 20/32 and distance of about 20/25 (Figure 1).3 A refractive error of -0.75D is optimal for maximal near and distance coverage via DOF.
PEARL. Soosan Jacob, MD, and her team based in India introduced the presbyopic allogenic refractive lenticule (PEARL) procedure to help avoid the pitfalls of corneal melt, implant fibrosis, opacification and haze associated with synthetic corneal implants (Figure 2).4
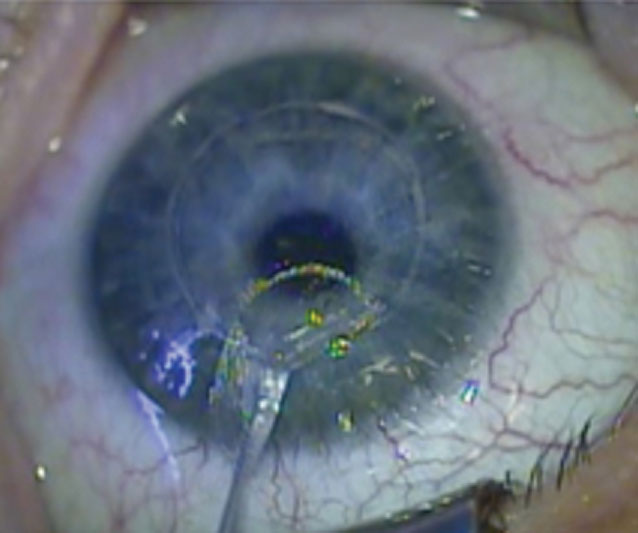 |
| Fig. 2. A donor lenticule harvested from a SMILE refractive patient is ready for trephining into a 1.0mm-diameter intrastromal implant. Photo: Anders Ivarsen, MD, PhD, Jesper Hjortdal, MD, PhD, DMSc. Click image to enlarge. |
A serologically tested donor lenticule harvested from the small-incision lenticule extraction (SMILE) surgery of a -2.00D to 2.50D patient is trephined to form a 1.0mm stromal disc that is implanted over the center of the pupil in a 120.0µm-deep femtosecond laser-created pocket.4 Once the cornea heals, the lenticule is invisible to the naked eye and results in a hyperprolate central cornea, creating the multifocal optic necessary for excellent near and far vision.4 The allograph is completely permeable to oxygen and corneal nutrients.4
VisAbility micro-insert (Refocus Group). This was conceived on the theory that presbyopia is primarily due to decreasing space between the lens equator and the ciliary muscle as the diameter increases with age.5 It consists of four 5.0mm-long polymethyl methacrylate segments implanted 4.0mm from the limbus between the extraocular muscles in the four quadrants of the eye (Figure 3).5
As a scleral treatment peripheral to the cornea, VisAbility completely avoids the eye’s optical pathway.5 Rather than offering a monovision treatment for presbyopia, it aims to provide natural, binocular vision without adverse effects on distance vision.5 FDA trial data revealed a 90% patient satisfaction rate with most patients reaching a UCNVA of 20/32 by three months after surgery.5
Disadvantages include extended postoperative conjunctival injection due to the conjunctival resection necessary to create the scleral tunnel and implant the micro inserts, prolonged optimal near visual acuity attainment until weeks or months after surgery and significant perioperative pain.5 The device is currently awaiting premarket approval from the FDA.5
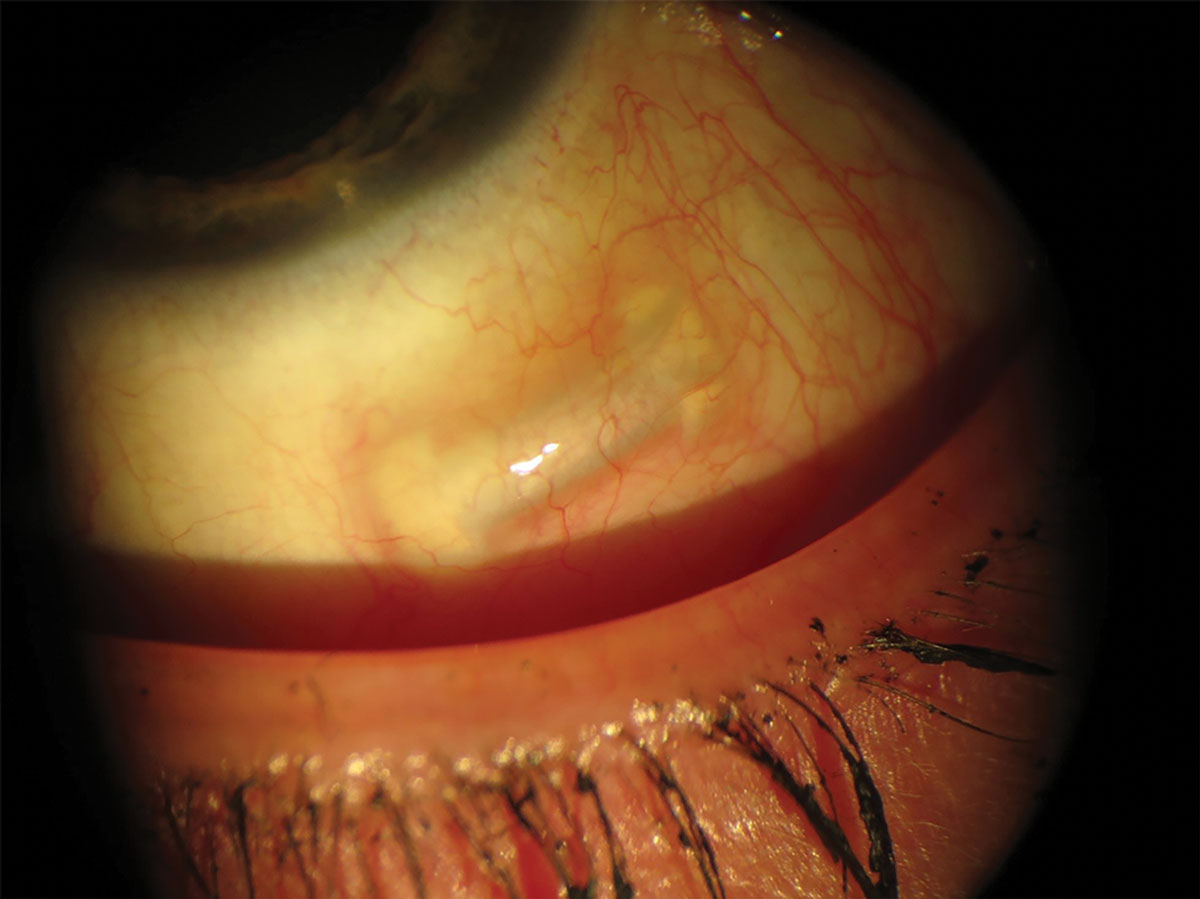 |
| Fig. 3. The VisAbility offers natural, binocular vision without affecting the eye’s optical pathway. Photo: Refocus Group. Click image to enlarge. |
LaserAce (Ace Vision Group). This is a less invasive, less surgical binocular treatment that does not alter the optics of the lens or cornea.6 It is based on the belief that scleral rigidity is the primary culprit in presbyopia.6 In a young eye, the sclera is more elastic and gives slightly with accommodative traction from the ciliary muscles. In an aging eye, the sclera is more rigid and resists movement associated with accommodation.
The procedure involves a series of scleral laser perforations using the company’s VisioLite Er-YAG laser.6 Four 5.0mm2 ablation matrices are applied in a diamond-shaped configuration to the four quadrants 4.0mm peripherally to the limbus.6 Each matrix of laser perforations overlies five key anatomical constituents of the accommodative mechanism, affording more elasticity to the sclera.6 As biomechanical efficiency increases, it translates to the lens during accommodation.6 The procedure has not yet entered into FDA investigational device exemption clinical trials.6
Ocufit (Sooft Italia). This stimulates the ciliary muscle to increase its potency so that it can overcome the higher resistance of the system associated with aging.7 It avoids altering the optics of the eye and aims to restore dynamic accommodation.7
The device consists of a 20.0mm scleral lens with four electrodes positioned 3.5mm from the limbus at the four quadrants.7 Electricity, which causes the ciliary muscle to spasm, is pulsed for two seconds with a rest time of six seconds for eight minutes.7 Four treatments are performed at two-week intervals.7 More extensive studies are needed to consider electrostimulation a contender for presbyopia treatment.
Corneal Procedures
Aside from monovision correction, there have been a number of attempts at presbyopia correction through multifocal corneal laser refractive procedures. PresbyLASIK describes a procedure that reshapes the cornea using standard laser refractive methods but alters the corneal laser ablation profile. This involves either making the peripheral cornea hyperprolate to create a central distance zone and a peripheral near zone or making the central cornea hyperprolate for a central near zone and a peripheral distance zone (Figure 4). Both techniques can be performed using LASIK or PRK.
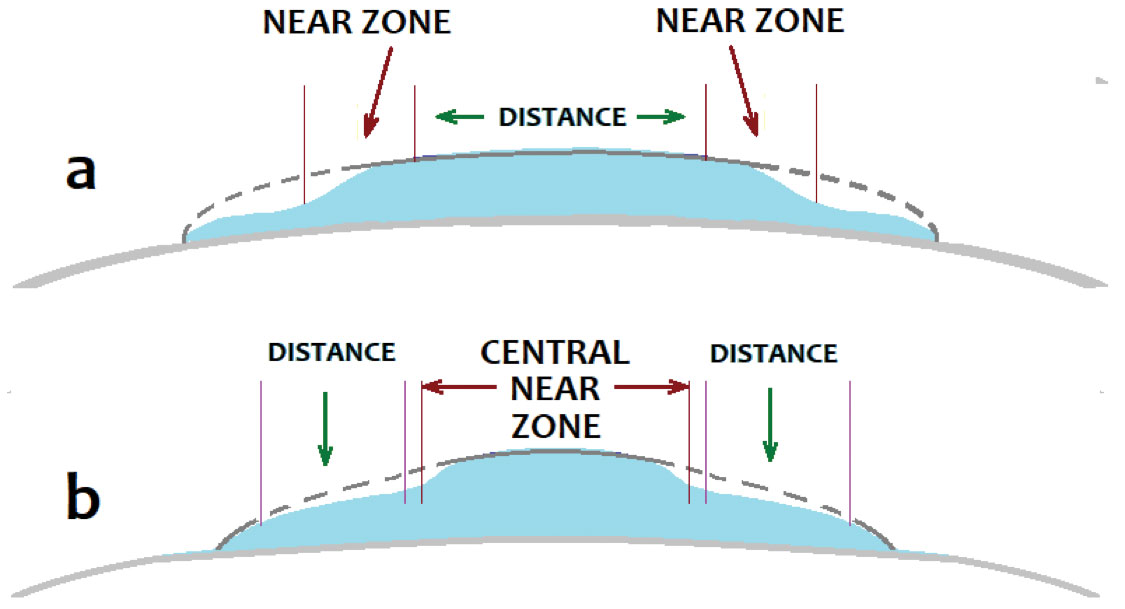 |
| Fig. 4. There are two configurations of PresbyLASIK: a near peripheral corneal annular zone with a distance central zone (a) and a near central corneal zone with a peripheral annular distance zone (b). Click image to enlarge. |
Supracor (Bausch + Lomb). This creates a variable-focus corneal profile with a 12.0μm elevation in the central 3.0mm, and provides a near addition power of approximately 2.00D.8 Peripheral to the central near portion is an aspheric annular zone, which provides intermediate and distance vision.8 It is best performed on hyperopic patients.8
Supracor outcomes vary depending on the technique’s magnitude and whether it is performed in tandem with a refractive procedure, as a singular presbyopic treatment or binocularly.8 While patients have generally been satisfied with their resulting near vision, distance vision disturbances have limited the procedure’s acceptance.8
Intracor (Bausch + Lomb). This employs a femtosecond laser to ablate concentric circles deep in the corneal stroma, inducing collagen shrinkage and causing a hyperprolate central near zone.9 Studies have demonstrated significant near vision improvement, but reductions in distance vision do occur and have precluded robust application.9 No clinical trials are currently in progress in the United States.9
Pharma Treatments
The medication realm may be home to the most encouraging class of treatment for presbyopic near vision loss. The aim in this case is threefold: soften the age-stiffened crystalline lens matrix to allow for recovery of natural dynamic accommodation with the ciliary body, produce miosis of the pupil to allow for expansion of optical DOF, and increase corneal tissue pliability to allow for rigid contact lens molding of the cornea and a multifocal shape profile.
Dioptin. This topical eye drop (UNR844, Novartis) is a lipoic acid-based, topically instilled prodrug that penetrates into the lens.10 A prospective double-blind FDA Phase I/II trial reported no serious adverse results and comparable comfort in participants and controls.10 After the 90-day dosing period, Dioptin-dosed subjects had achieved a distance-corrected near visual acuity (DCNVA) of 20/22 and controls, 20/40.10 The near acuity improvement persisted through the 301-day follow-up.10
TVT. Yolia Health’s True Vision Treatment (TVT) is a seven-day combo therapy involving an eye drop to make the cornea more malleable and a cornea-shaping contact lens designed for eight hours of wear.11 The company claims the molding effect lasts more than seven months.11 The twofold nature of this treatment has complicated the FDA trial process.11 However, results have been encouraging, with reports of binocular UCNVA improving from 20/80 to 20/40.11 Distance acuity was not adversely affected, but it is unknown whether aberrations typical of multifocals caused visual disturbances.11
Liquid Vision. These eye drops (PRX-100, Presbyopia Therapies) encourage pupil miosis to improve both near and far visual acuity via DOF expansion.12 In younger presbyopes, the myopic shift of the crystalline lens associated with the ciliary spasm can result in reduction of distance visual acuity. This drop is meant to solve these problems with its preparation of aceclidine.12
The FDA Phase IIb study found that miosis took place about 30 minutes after eye drop instillation, with 47.2% of eyes gaining at least three lines of DCNVA and 91.7% gaining at least two.12 The drug’s effect lasted as long as seven hours, and there was no discomfort or adverse effect on distance visual acuity.12 The medication will enter FDA Phase III clinical trials in the first half of 2020.12
PresbiDrops. This drop (CSF-1, Orasis) combines a parasympathomimetic with an NSAID in an oil-based vehicle to preclude discomfort due to ciliary spasm and minimize the risk of uveitis.13 The Phase IIb clinical trial met the three-line improvement criteria for DCNVA and achieved good comfort with no significant adverse effect on distance vision.13 The company claims that the drug has a fast onset of action and its effects are long-lasting and is now recruiting for FDA Phase III clinical trials.13
Oxymetazoline. This drug (AGN-199201, Allergan) is a vasoconstriction decongestant, a direct-acting alpha-1 adrenergic agonist and alpha-2a adrenergic partial agonist, traditionally used to treat sinus congestion and conjunctival hyperemia.14 In the Phase II trial, about 70% of subjects had at least a two-line improvement in UCNVA.14 Allergan is currently recruiting for Phase III trials for two preparations (AGN-190584 and AGN-199201), individually and in combination with each other.14
Many investigators are in the process of testing drugs and combination therapies to improve near vision, nearly all of which involve pupillary miosis. Most are not in the FDA pipeline yet, but all have achieved similar outcomes in terms of time to onset and duration of effect.15,16 International examples include FOV Tears produced by Luis Felipe Vejerano, MD, Método Benozzi by Jorge Benozzi, MD, and PresbiPlus by Roberto Pinelli, MD.15,16
Lens Replacement
Intraocular lenses (IOLs) are not considered a treatment for presbyopia per se, but many ophthalmologists who lens replacement surgery on patients without cataracts by substituting the healthy crystalline lens with an IOL to correct the refractive error while providing near vision, intermediate vision or both. This surgery is also referred to as clear lens replacement or refractive lens exchange (RLE). Three types of IOL configurations can be employed in RLE:
Monofocal monovision. Monofocal IOLs (spherical or spherocylindrical) are geared toward patients who have had success with contact lens monovision. However, monofocal IOLs have a minimal DOF, so it must be decided prior to surgery whether intermediate or near vision is more important to the patient based on their working distance demands.
EDOF, trifocal IOLs. Extended DOF (EDOF) and trifocal IOLs are a new generation of IOLs that provide clearer vision at all working distances. Sometimes promoted as presbyopia-correcting IOLs, these lenses can be used in a modified monovision configuration or they can be binocularly employed. For the most part, they have largely replaced multifocals as the ultimate choice for continuous vision at a full range of distances.
FDA-approved in 2016, the Tecnis Symfony EDOF IOL (Johnson & Johnson) has a lens surface that carries achromatic diffractive grating elements called echelettes, which extend DOF and concurrently correct chromatic dispersion.17 Rather than prismatically splitting light to produce a second near focal point like multifocals, echelettes offer a more continuous range of visual working distances.17 Reduced chromatic dispersion results in higher contrast sensitivity, reduction of glare and halos and higher visual quality.17 Near vision can be compromised, so patients may occasionally need assistance from near spectacles for close targets.17
The Acrysof IQ PanOptix trifocal IOL (Alcon), which received FDA approval in August 2019, has a trifocal diffractive surface.18 Its three dioptric powers are targeted for 40.0cm, 60.0cm and infinity, and it is available with astigmatic correction.18
Other EDOF and trifocal IOLs in development that have found success internationally are the AT Lisa trifocal IOL (Carl Zeiss Meditec), the Alsafit trifocal VF lens (Alsanza), the FineVision Triumf trifocal EDOF lens (PhysIOL) and the Mini Well Ready EDOF lens (SiFi Medtech).19,20 The Mini Well Ready is unique in that it has wavefront-guided progressive optics without diffractive lens zones, a peripheral monofocal distance optic, a middle distance optic with oppositely-signed spherical aberration and a central distance zone.20 This combination precludes halos and provides a continuous range of vision.20
Accommodating IOLs. The race is on for a lens that will fit into the capsular bag and re-establish normal dynamic accommodation. This is what accommodating IOLs (AIOLs) aim to do.
The only AIOLs approved so far in the US are the Crystalens AO and HD (Bausch + Lomb).21,22 The Crystalens has articulating haptics that are supposed to bend on accommodative effort and translate the optic forward.21,22 Research, however, has demonstrated that it does not accommodate as earlier stated; rather than the 1.50D to 1.90D theorized by a 1.0mm displacement of the optic with accommodation, forward translation of the optic has been measured at roughly 0.4mm and has even been observed to tilt backward, creating aberrations that would account for a near increase in DOF.21,22
There are many AIOLs not yet approved in the United States that show true optical change with accommodative effort.
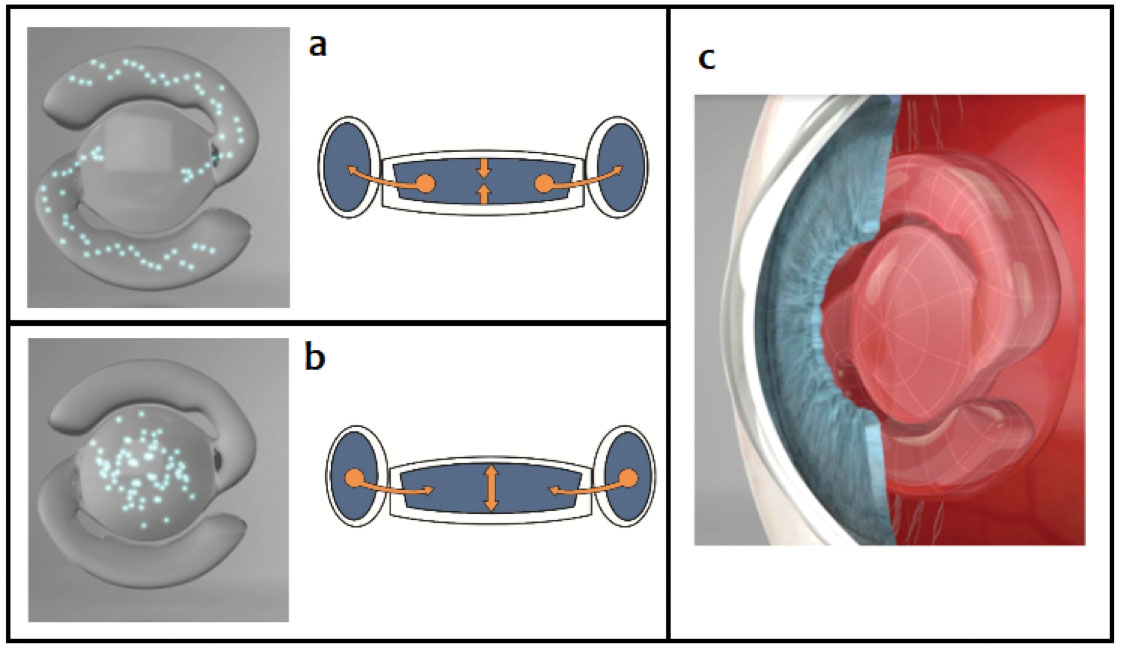 |
| Fig. 5. In the un-accommodated state (a), a minute amount of fluid streams from the central optic into the peripheral balloon haptics of the lens, and the distance power of the optic is obtained. Upon accommodation and radial contraction of the lens capsule by the ciliary body (b), the fluid streams into the central optic, increasing its plus power for near vision provided by the FluidVision AIOL (c). Photo: Adapted from Ophthalmology Innovation Summit. Click image to enlarge. |
The FluidVision AIOL (Alcon) is promoted as the first true shape-changing, fluid-driven AIOL.23 The lens has three main components: (1) a flexible central optic reservoir, (2) flexible pontoon-like haptics that also serve as reservoirs and (3) about 30µL of fluid (Figure 5).23 Its method of action is based on the principle of ciliary compression; accommodative effort causes the ciliary body to compress the haptics, which causes fluid to stream out to the central optic.23 As the central optic fills, the plus power of the lens increases, focusing the IOL for near.23 Theoretically, graded action from the ciliary body should be able to provide a continuous range of focus for the patient.23
A study reported good visual acuity at every distance, with mean distance vision at 20/20, intermediate vision at 20/20 to 20/25 and near vision at 20/20 to 20/27.23 Accommodation was measured at a mean of 2.00D, and accommodative amplitudes as high as 5.00D were achieved with accommodative effort.23 Alcon named the newest version the NextGen 20/20 and is currently undergoing an international multicenter clinical trial.23
Certain AIOL designs depend on the compressive action of the ciliary muscle to produce axial movement of the IOL optic, which has proved problematic.24 In addition, IOLs positioned inside the capsular bag have been subject to capsular fibrosis, shrinkage and stenosis of the haptics, compounding the loss of IOL functionality over time.24
The Lumina AIOL (AkkoLens) went in a different direction, using an opposing pair of optics called Alvarez lenses—freeform progressive lenses that vary the dioptric power through the pair when the lens elements move transversely to each other at a 90o angle to the pupillary axis (Figure 6).25 When the ciliary body compresses the AIOL haptics with near accommodative effort, elements of the lens transverse one another with the net optical combination increasing the plus power of the lens.25 For distance vision, the ciliary body relaxes and decompresses the haptics, allowing the lens elements to realign.25
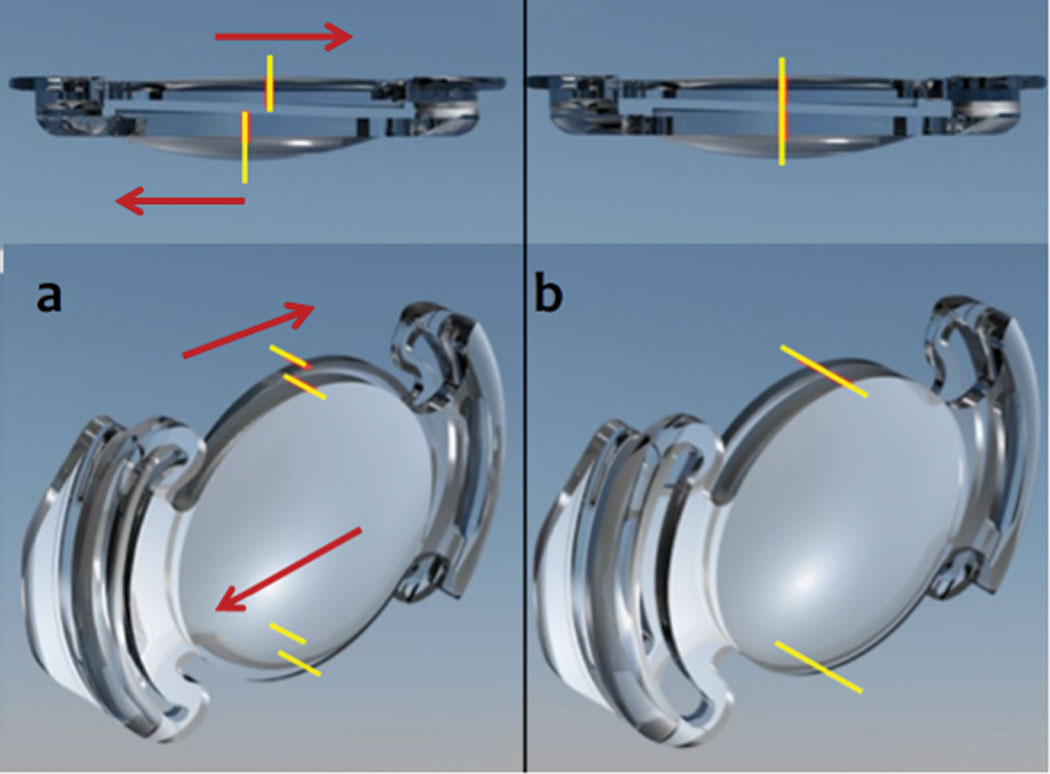 |
| Fig. 6. As the ciliary body of the Lumina AIOL compresses the lens haptics (a), the lenses translate in apposition to each other, increasing plus power. With relaxation of the ciliary body (b), the lenses line up with the appropriate distance power. Photo: Adapted from AkkoLens. Click image to enlarge. |
Rather than being located in the bag and subjected to fibrosis, the Lumina AIOL is positioned at the sulcus plane where the ciliary body muscle contacts the opposing elements of the lens, moving them transversely and engendering the accommodative myopic shift.25 Breaking up the capsule can overcome restriction by capsular bag fibrosis.25
While a study found a positive accommodative response to a stimulus—up to 4.50D—in the Lumina AIOL compared with an absent response in a monofocal IOL, there are issues with accommodative response variability from patient to patient.25
The Juvene AIOL (LensGen) uses a two-part system that can be inserted into a smaller incision and assembled in the eye: a peripheral carrier that fills the capsular bag and a central fluid-filled optic that deforms to become more prolate as the carrier is compressed by the ciliary body. The device is simple and relatively clear of higher-order aberrations.26
Data from clinical trials in Mexico and the Dominican Republic indicate that patients can maintain 2.50D of accommodation and achieve up to 3.00D.27 Another study reported that about 50% of Juvene-implanted patients can achieve a DCNVA of 20/32 and 70%, 20/40.27
IOLs that employ electro-optics and contain artificial intelligence software sense pupil constriction due to accommodation—distinct from the pattern and speed of constriction due to light reaction. Electro-optical IOLs may be incorporated into the long-term outlook on IOL technologies, but far simpler solutions exist that do not require nearly as much hardware or software.
In the near future, it is likely that a pharmaceutical solution will be the first big wave of treatment, and, in that case, a combination approach would be the most effective. Years from now, these drops may be available over-the-counter on shelves in pharmacies next to dollar readers. Presbyopic surgical methods are also always developing, further encouraging the rise of combination therapies. Patients over 60 will undergo RLE more often as procedures and AIOL technologies improve and receive FDA clearance. Despite the emergence and probable dominance of AIOLs, it is unlikely that multifocal and EDOF IOLs will go away, as the quality of vision from these lenses continues to improve with each generation.
Just as the exact cause of presbyopia is not entirely known, neither is the ideal treatment for the condition—one that reverses the presbyopic process and restores natural accommodation with the native crystalline lens. We can only hope that when one does emerge, it is affordable and accessible to the millions of people who experience the handicap of near vision loss around the world.
1. Duane A. Studies in monocular and binocular accommodation with their clinical applications. Am J Ophthalmol. 1922;5(11):865-77. 2. Markets Insider. Myopia & presbyopia treatment market size worth $28 billion by 2026: Grand View Research, Inc. markets.businessinsider.com/news/stocks/myopia-presbyopia-treatment-market-size-worth-28-billion-by-2026-grand-view-research-inc-1028570106. October 2, 2019. Accessed February 25, 2020. 3. Vukich JA, Durrie DS, Pepose JS, et al. Evaluation of the small-aperture intracorneal inlay: three-year results from the cohort of the U.S. Food and Drug Administration clinical trial. J Cataract Refract Surg. 2018;44(5):541-56. 4. Jacob S, Kumar DA, Agarwal A, et al. Preliminary evidence of successful near vision enhancement with a new technique: PrEsbyopic Allogenic Refractive Lenticule (PEARL) corneal inlay using a SMILE lenticule. J Refract Surg. 2017;33(4):224-9. 5. Baitch L, Schanzlin D, Iskander D. One-year post-operative wavefront and refractive map changes following scleral implant surgery. Presented at the 15th International Congress on Wavefront & Presbyopic Refractive Correction; 2014; Dana Point, California. 6. Hipsley A, Hall, B, Rocha KM. Long-term visual outcomes of laser anterior ciliary excision. Am J Ophthalmol Case Rep. 2018;10:38-47. 7. Gualdi L, Gualdi F, Rusciano D, et al. Ciliary muscle electrostimulation to restore accommodation in patients with early presbyopia: preliminary results. J Refract Surg. 2017;33(9):578-83. 8. Ryan A, O’Keefe M. Corneal approach to hyperopic presbyopia treatment: six-month outcomes of a new multifocal excimer laser in situ keratomileusis procedure. J Cataract Refract Surg. 2013;39(8):1226-33. 9. Thomas BC, Fitting A, Khoramnia R, et al. Long-term outcomes of intrastromal femtosecond laser presbyopia correction: 3-year results. Br J Ophthalmol. 2016;100(11):1536-41. 10. Stein JM, Robertson, SM, Evans DG, et al. An observational follow-up study assessing the long-term effects of bilaterally dosed topical lipoic acid choline ester eye drops for the treatment of presbyopia. Inv Ophthalmol Vis Sci. 2017;58(8):330. 11. Plenilunia Salud Mujer. Presenting a non-surgical procedure for presbyopia, which represents an option for patients with tired eyesight. plenilunia.com/noticias-2/presentan-procedimiento-no-quirurgico-para-presbicia-representa-opcion-para-pacientes-con-vista-cansada/55306. July 5, 2018. Accessed February 25, 2020. 12. Lipner M. EyeWorld. A unique drop. www.eyeworld.org/article-a-unique-drop. October 2014. Accessed February 25, 2020. 13. ClinicalTrials.gov. Safety, tolerability, and efficacy of PresbiDrops (CSF-1), a topical ophthalmic drug for presbyopia. clinicaltrials.gov/ct2/show/NCT02745223. April 20, 2016. Accessed February 25, 2020. 14. ClinicalTrials.gov. A Phase 3 efficacy study of AGN-190584 in participants with presbyopia. clinicaltrials.gov/ct2/show/study/NCT03804268?term=Allergan%2C+agn-190584&draw=2&rank=2. January 15, 2019. Accessed February 25, 2020. 15. Vargas V, Vejarano F, Alió JL. Near vision improvement with the use of a new topical compound for presbyopia correction: a prospective, consecutive interventional non-comparative clinical study. Ophthalmol Ther. 2019;8(1):31-9. 16. Healio. Clinicians anticipate treating presbyopia with eye drops. www.healio.com/optometry/optics/news/print/primary-care-optometry-news/%7B47bf090e-50e5-4c49-aa96-d49d9843d2e1%7D/clinicians-anticipate-treating-presbyopia-with-eye-drops?page=7. August 2017. Accessed February 25, 2020. 17. Cochener B, Boutillier G, Lamard M, et al. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34(8):507-14. 18. Lawless M, Hodge C, Reich J, et al. Visual and refractive outcomes following implantation of a new trifocal intraocular lens. Eye Vis (Lond). 2017;4:10. 19. Esteve-Taboada JJ, Domínguez-Vicent A, Del Águila-Carrasco AJ, et al. Effect of large apertures on the optical quality of three multifocal lenses. J Refract Surg. 2015;31(10):666-76. 20. Savini G, Schiano-Lomoriello D, Balducci N, et al. Visual performance of a new extended depth-of-focus intraocular lens compared to a distance-dominant diffractive multifocal intraocular lens. J Refract Surg. 2018;34(4):228-35. 21. Marcos S, Ortiz S, Pérez-Merino P, et al. Three-dimensional evaluation of accommodating intraocular lens shift and alignment in vivo. Ophthalmology. 2014;121(1):45-55. 22. Pérez-Merino P, Birkenfeld J, Dorronsoro C, et al. Aberrometry in patients implanted with accommodative intraocular lenses. Am J Ophthalmol. 2014;157(5):1077-89. 23. DeBoer CM, Lee JK, Wheelan BP, et al. Biomimetic accommodating intraocular lens using a valved deformable liquid balloon. IEEE Trans Biomed Eng. 2016;63(6):1129-35. 24. Alió JL, Ben-Nun J. Study of the force dynamics at the capsular interface related to ciliary body stimulation in a primate model. J Refract Surg. 2015;31(2):124-8. 25. Alió JL, Simonov A, Plaza-Puche AB, et al. Visual outcomes and accommodative response of the Lumina accommodative intraocular lens. Am J Ophthalmol. 2016;164:37-48. 26. Devgan U, Kohnen T, Donnenfeld E, et al. Dual-optic curvature-changing modular-design presbyopic IOL. Presented at the 37th Congress of the European Society for Cataract and Refractive Surgery; 2019; Paris, France. 27. Pepose JS, Burke J, Qazi M. Accommodating intraocular lenses. Asia Pac J Ophthalmol (Phila). 2017;6(4):350-7. |


