Pathologic Causes of Irregular Astigmatism
Thin is in—but not in the cornea. Here’s a rundown of non-inflammatory corneal thinning disorders that lead to irregular astigmatism.
By Thomas Stokkermans, OD, PhD
Release Date: June 15, 2019
Expiration Date: June 15, 2022
Estimated time to complete activity: 1 hour
 |
Jointly provided by Postgraduate Institute for Medicine and Review Education Group.
Educational Objectives: After completing this activity, the participant should be better able to:
- Discuss the prevalence of astigmatism in the population and how the changes that occur in astigmatism throughout life may provide some clues to the causes of astigmatism.
- Identify the involvement and interaction of genetic and environmental factors in the development of irregular astigmatism.
- Explain how corneal degenerations—non-inflammatory corneal thinning disorders—result in irregular astigmatism.
- Describe the presenting signs and features, as well as the natural progression of keratoconus and the non-inflammatory corneal thinning disorders.
- Review the most effective options—particularly surgical options (including corneal crosslinking)—for correcting keratoconus and other non-inflammatory thinning disorders.
Target Audience: This activity is intended for optometrists engaged in the care of patients with irregular astigmatism.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Review Education Group. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Thomas Stokkermans, OD, PhD, Case Western Reserve University.
Credit Statement: This course is COPE approved for 1 hour of CE credit. Course ID is 62684-AS. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Stokkermans: Nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The Review Education Group planners, managers and editorial staff have nothing to disclose.
When a refraction doesn’t produce visual acuity of 20/20 or leaves the patient with glare and ghosting, and your subsequent slit-lamp exam doesn’t indicate an overt cause, irregular astigmatism should be at the top of your differential diagnosis list.
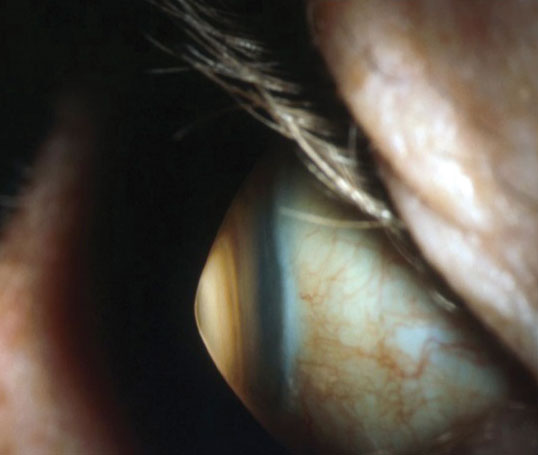 |
| Keratoconus is associated with central corneal thinning resulting in corneal ectasia. Photo: Jonathan Lass, MD. Click image to enlarge. |
In these cases, corneal topography, pachymetry, retinoscopy with careful observation of the reflex, a rigid gas permeable contact lens over-refraction, wavefront aberrometry or anterior segment optical coherence tomography (AS-OCT) can all help you identify irregular astigmatism as the culprit.
While the crystalline lens may be one source of the problem, irregular astigmatism is more often caused by the cornea. Of the corneal etiologies, keratoconus is the most common cause of primary irregular astigmatism (i.e., not caused by extraneous causes such as surgery or contact lens wear) (Table 1).1
To know when high amounts of astigmatism, myopia and anisometropia are likely caused by keratoconus or other non-inflammatory thinning disorders, you must have a working knowledge of the development and epidemiology of refractive error. This article can help you diagnose and manage patients with irregular astigmatism.
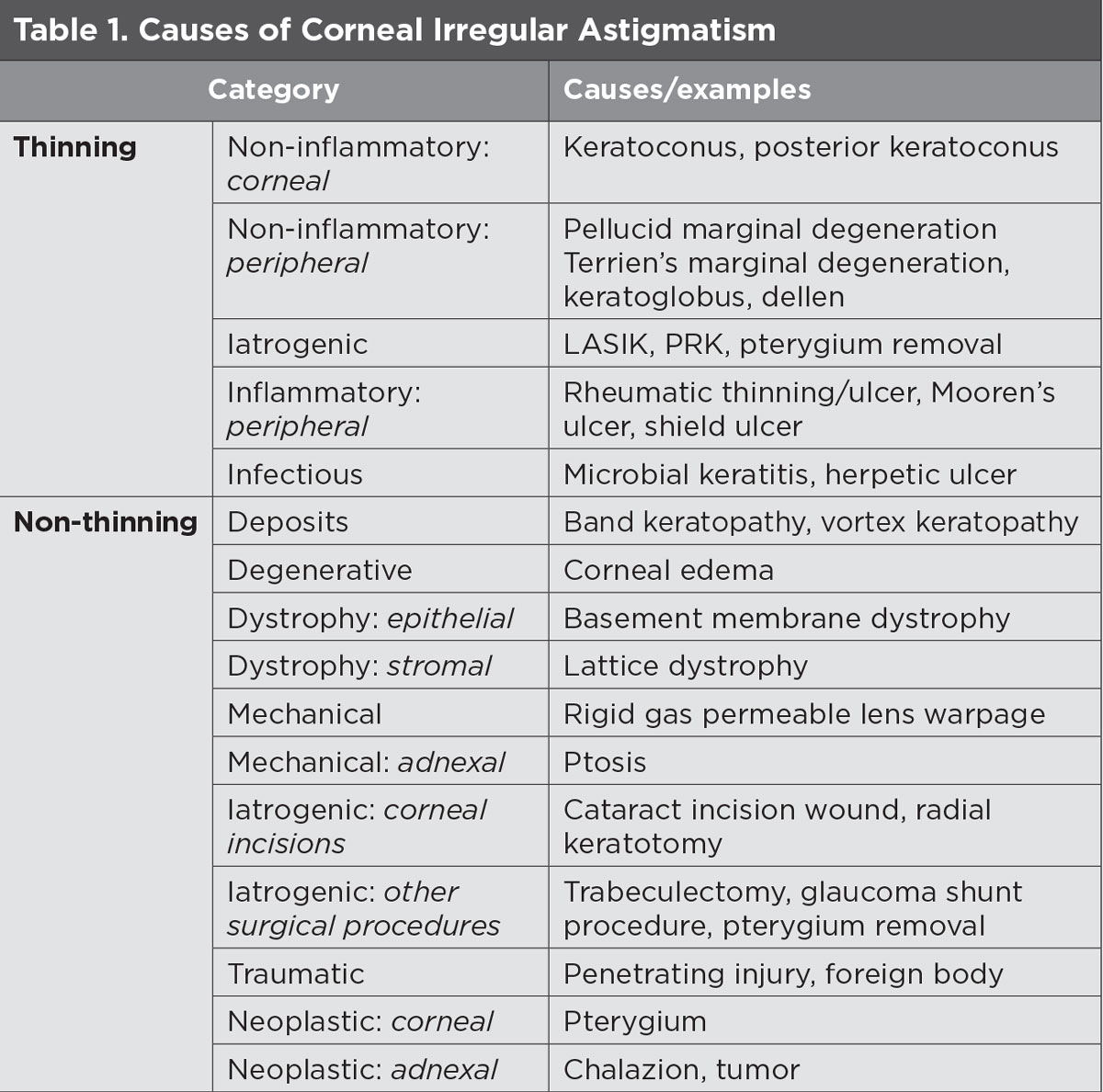 |
| Click table to enlarge. |
Refractive Errors
Refractive errors at birth are variable, decreasing until age six, when the highest degree of emmetropia is present.2,3 This applies to astigmatism as well. The average amount of astigmatism at birth is 3D and is reduced to just 1D by age five.
However, not all children have a reduction in their refractive errors, as patients with high amounts of with-the-rule or low amounts of against-the-rule astigmatism will likely have significant residual astigmatism later in life.2,3 This applies to anisometropia as well—while it is common to have some degree of anisometropia during the first few years of life, children with higher amounts of anisometropia at a young age are more likely to have it throughout their life.2,3
Non-pathologic myopic progression can be divided into four categories: congenital (present at birth, generally no emmetropization occurs), youth onset (occurs between six years and early teens), early-adult onset (occurs between the ages of 20 and 40) and late-adult onset (occurs after age 40).2,3
Family history strongly affects the risk for youth onset and early-adult onset myopia. For example, one parent with myopia increases the odds of their child developing myopia by threefold, while two myopic parents increases the odds almost eightfold.4 As such, concern for corneal thinning is greatest in rapidly increasing youth onset myopia or early-adult onset myopia for children with parents with myopia.5
Regular astigmatism. Astigmatism of 0.5D and higher is found in 15% of children and 40% of adults worldwide and is the most prevalent refractive error compared with myopia and hyperopia.6 Regular astigmatism of more than 3D makes up a small percentage of astigmatic patients, with one study finding one in 20 individuals affected.7
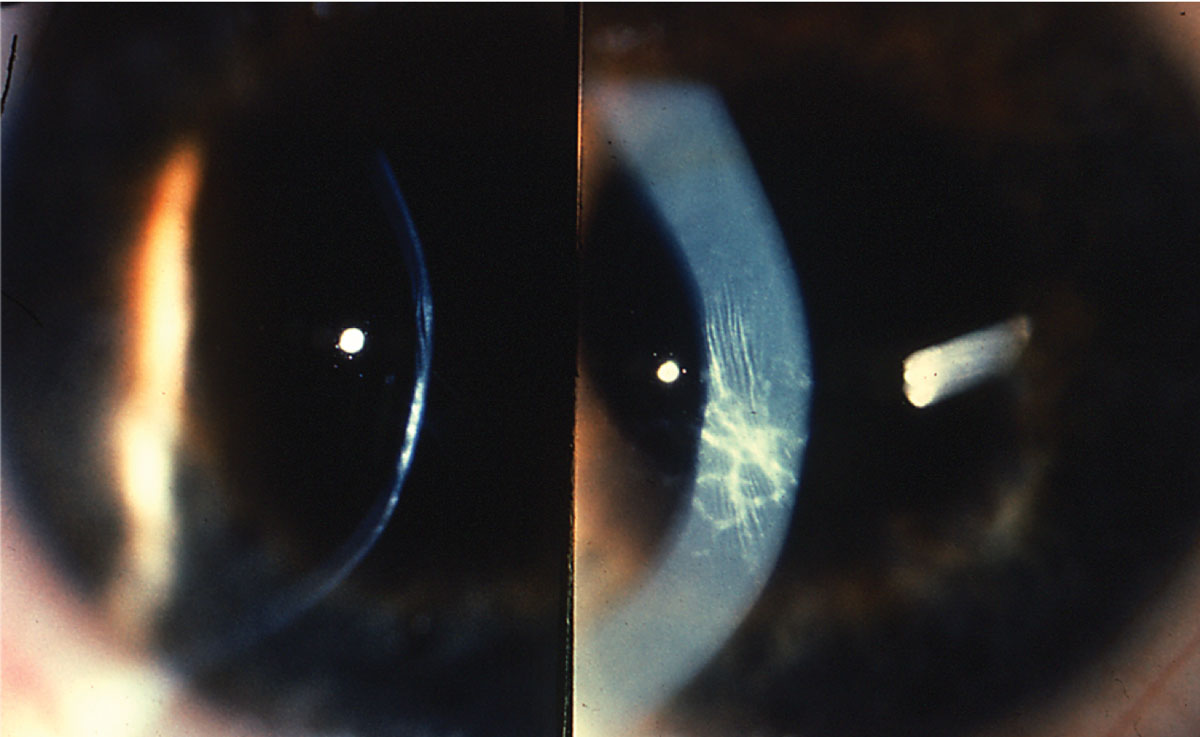 |
| Apical scarring, together with Vogt striae, is a sign of moderate keratoconus. Photo: Jonathan Lass, MD. Click image to enlarge. |
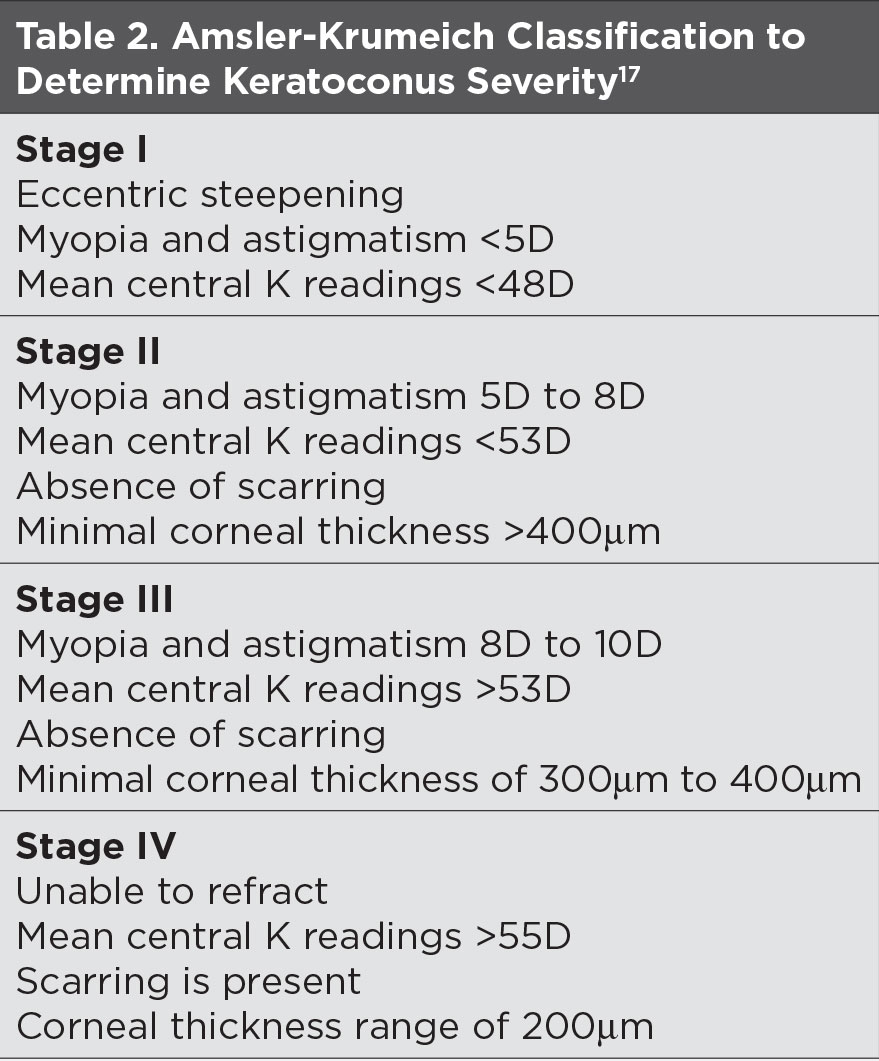 |
| Click table to enlarge. |
Unlike myopia, astigmatism in a teenager is much less dependent on family history, even though research suggests an autosomal dominant inheritance pattern.8,9
Asymmetry of astigmatism between the eyes is also uncommon (<20%) with asymmetry decreasing as astigmatism increases.10 The axis of astigmatism follows mirror or direct symmetry in four out of five patients.10 So, clinicians should have a high index of suspicion for ectasia in patients with asymmetrical high amounts of astigmatism.
Irregular astigmatism. This is a combination of higher-order aberrations such as coma, trefoil and quadrafoil, each of which can be quantified in terms of Zernike polynomials. Aberrometry allows us to quantify these different types of aberrations, and research shows the detection of higher amounts of vertical coma and overall amount of higher-order aberrations are good predictors of keratoconus.11
Once you’ve made the diagnosis of irregular astigmatism and have ruled out inflammatory corneal thinning, it’s time to consider the causes of non-inflammatory corneal disorders and potential treatment strategies.
Non-inflammatory Corneal Thinning Disorders
Keratoconus is the most common non-inflammatory corneal thinning disorder, but it’s certainly not the only one. Other thinning disorders—including pellucid marginal degeneration (PMD), keratoglobus, posterior keratoconus and Terrien’s marginal degeneration (TMD)—may closely resemble keratoconus, so a thorough examination is necessary in each case.
Keratoconus (KCN). This condition occurs in one in 500 to 2,000 people.1,12,13 Men and women are equally likely to develop KCN, and it’s also more common in Asians.13 Research estimates genetics contribute the majority (60%) of the risk.14 Patients with a first-degree relative with KCN have an increased risk of one in 30, and in turn, patients with diagnosed KCN have a one in six to one in 16 chance of having a family member with the diagnosis.12,13
Besides genetics, common risk factors determined in the Collaborative Longitudinal Evaluation of Keratoconus study include eye rubbing (50%) and atopy (53%).15 Studies have also associated it with some systemic diseases, including Down syndrome and Leber’s congenital amaurosis.12
Keratoconus generally develops in the second decade of life, along with myopia and regular astigmatism, and stabilizes in the fourth decade. It’s associated with progressive high myopia, astigmatism, anisometropia and reduced visual acuity. It often develops asymmetrically—one in seven patients has KCN in strictly one eye.12
Clinical signs of KCN include a full or partial circle of corneal hemosiderin deposition that’s best visualized with cobalt blue illumination (Fleischer ring), endothelial vertical lines (Vogt striae), increased visibility of corneal nerves, corneal thinning and scarring, a V-shaped distortion of the lower lid on downgaze (Munson’s sign), a conical-shaped reflection on the nasal cornea when a light is shone from the temporal cornea (Rizzuti’s sign), a round droplet-shaped reflection observed in retro-illumination of the pupil (Charleaux’s “oil droplet” sign), a scissored reflex with retinoscopy and, rarely, a painful sudden onset of stromal edema and opacification that partially clears in weeks (hydrops).12
| Is Keratoconus Truly a Non-inflammatory Disorder? Keratoconus is considered a non-inflammatory corneal disorder because it develops in the absence of neovascularization and corneal infiltrates. But at the same time, a significant body of literature supports a role of inflammation in the development and progression of the disease.1,2 The association between atopy, elevated immunoglobulin E and keratoconus was made more than half a century ago.2 More recently, studies have found that patients with keratoconus have more inflammatory mediators in their tears.1 Also, eye rubbing associated with eye irritation and other mechanical insult to the cornea such as rigid gas permeable contact lens wear, and the inflammation caused by this, may actually be the direct cause of keratoconus. In fact, in atopic patients keratoconus occurs more often on the side of the dominant hand.2 What other evidence do we have? It seems that much of it comes down to an inadequate balance of inflammatory mediators that may make some corneas more sensitive to mechanical insult. For instance, proteolytic enzymes and prostaglandins are upregulated, which promotes collagen degradation and inhibits collagen synthesis—processes that may be responsible for thinning in keratoconic corneas. Meanwhile, upregulation of pro-inflammatory cytokines and other molecules can directly cause corneal cell death. And another group of mediators, including protease inhibitors and anti-inflammatory cytokines, are downregulated in keratoconus. Downregulation of this group results not only in upregulation of the other mediators, but also directly causes oxidative stress, collagen degradation, cell death and generalized reduction in immune functions. Still, these results leave us with the question: Why do we find virtually no clinical and histological evidence of inflammation in patients with keratoconus? One possibility is that while both inflammation and keratoconus are present at the same time, there may be no causal relationship between the two.
|
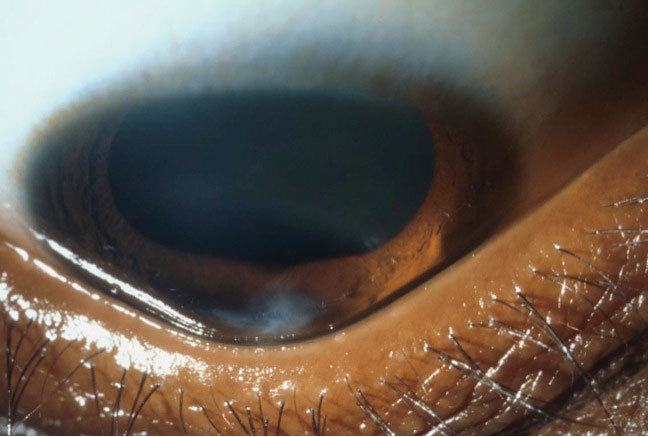 |
| The triangular deformation of the lower eyelid contour when looking down, or Munson’s sign, is an indicator of advanced keratoconus. Photo: Jonathan Lass, MD. Click image to enlarge. |
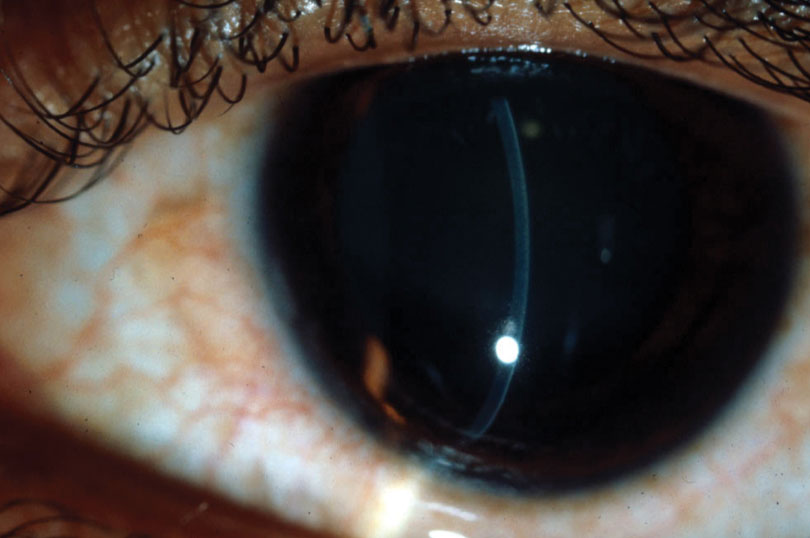 |
| In keratoglobus, peripheral thinning of the cornea is associated with a large area of steepening. Photo: Jonathan Lass, MD. Click image to enlarge. |
In the absence of overt signs, manual keratometry, topography, AS-OCT, pachymetry and aberrometry can help diagnose KCN while aiding in disease monitoring (Table 2).16 These tests can detect “forme fruste” keratoconus in asymptomatic patients who are 20/20.
Two well established benchmarks of KCN are a central keratometry reading of more than 47.2D and a difference between the eyes of 0.92D or a 1.4 inferior minus superior (I-S) ratio.18 The I-S ratio compares corneal curvature 3mm superior and inferior to the apex of the topographical map.
Another indication of KCN is when pachymetry deviates about 10% from the expected corneal thickness (550μm) and the thinnest point is not located within the central 1mm or there is asymmetry in corneal thickness between the two eyes.19 The eyelid rubs on the corneal epithelium about 16,000 times a day, flattening the anterior surface in keratoconus. So, the posterior corneal curvature, and 10μm or more of elevation compared with the expected position, is a better indicator than anterior curvature in early KCN.20
Classification of the cone based on size of ectasia is standard practice; the three categories are “nipple cone” (less than 5mm), “oval cone” (greater than 5mm) and keratoglobus (three quarters of cornea affected).
Most cases of keratoconus can be treated with glasses and contact lenses. But in some cases, scarring, excessive corneal distortion and steepening may make it impossible to correct vision with contact lenses. The risk for scarring is highest in patients younger than age 20, those with corneas steeper than 52D, with corneal staining and contact lens wearers.21 The lifetime risk of patients with keratoconus requiring corneal transplantation is between one in five to one in 10.22
Pellucid marginal degeneration. Unlike keratoconus, this has a male predilection and causes thinning of the peripheral inferior cornea approximately 2mm from the limbus.23 A Fleischer ring or Vogt striae are not common. Because both inferior KCN and PMD may reveal a “crab’s claw” or “kissing doves” topographic pattern, and because many topography units don’t measure beyond the central 9mm of the cornea, full-diameter pachymetry is required to differentiate these two related conditions.24
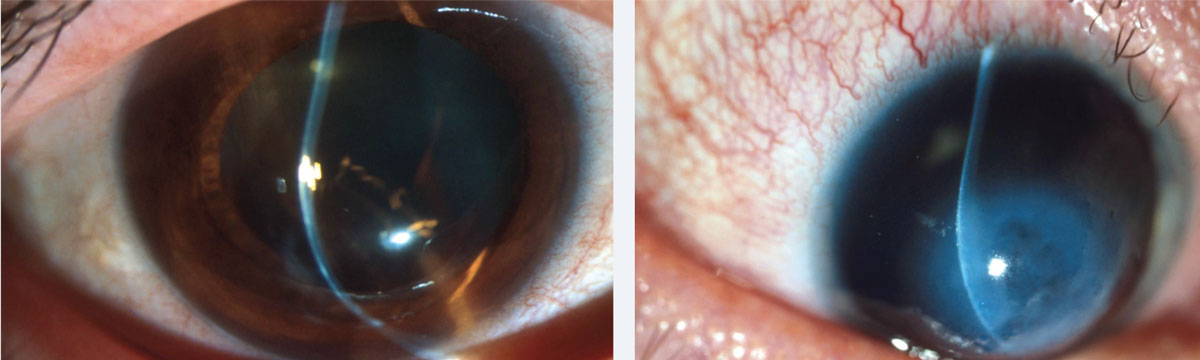 |
| At left, inferior steepening of the cornea associated with thinning at 2mm from the limbus is the hallmark of PMD. At right, corneal hydrops in a patient with Down syndrome and advanced keratoconus. Photos: Jonathan Lass, MD. Click images to enlarge. |
Optical densitometry using a Scheimpflug camera, while a technique not available in the average optometry office, can also be used to differentiate KCN from PMD. Both central and inferior KCN have elevated densitometry in the central cornea only, while PMD has elevation in the peripheral cornea as well. Correct diagnosis is important, as PMD tends to be diagnosed later than KCN, in the second to fifth decade of life, and is generally less severe.
Progressive, against-the-rule irregular astigmatism may result in the need for rigid contact lenses or surgery. Due to the peripheral nature, both contact lens and surgical options are often modified from those used for KCN. Corneal collagen crosslinking may be used to stabilize PMD.25
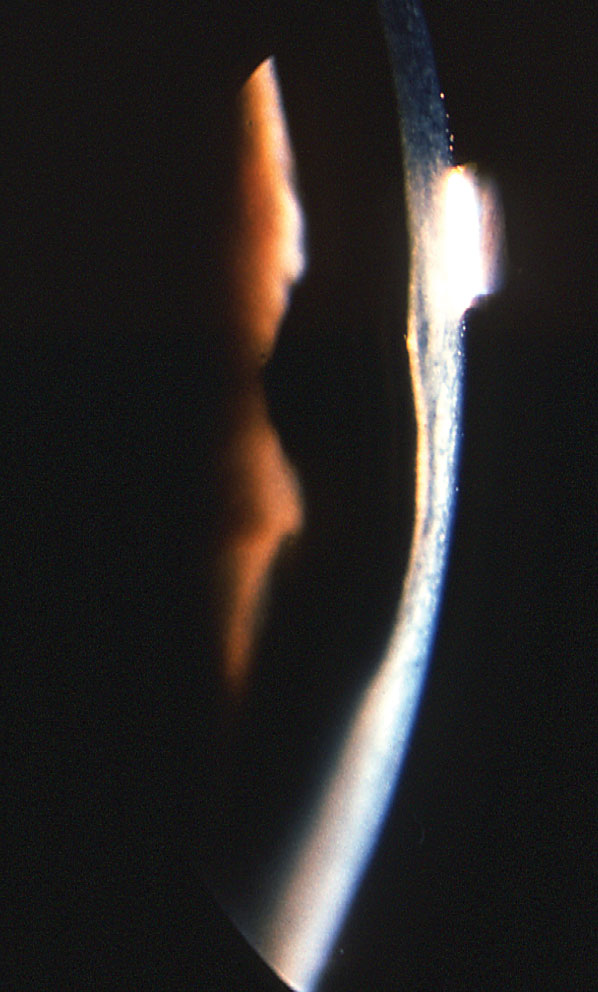 |
| In posterior keratoconus, the posterior cornea is thin and the posterior float is significantly elevated without increased curvature of the corneal anterior surface. Photo: Jonathan Lass, MD. Click image to enlarge. |
Keratoglobus. This condition presents with clear, diffuse, progressive bilateral corneal thinning, especially in the periphery.26 It results in high myopia, irregular astigmatism, scarring in the case of hydrops and, rarely, globe rupture.26 It can be congenital or acquired. Congenital cases can be associated with blue sclera and connective tissue disorders such as Ehlers-Danlos syndrome, Marfan syndrome and osteogenesis imperfecta.26 Vision can often be corrected with spectacle wear. Advanced cases of keratoglobus with more thinning and irregular astigmatism can be treated with contact lenses and specialized corneal transplantation techniques such as tuck-in lamellar keratoplasty.
Posterior keratoconus. This is generally congenital, non-progressive, often unilateral and presents with an abnormal posterior and normal anterior corneal curvature.27,28 When congenital, a specific abnormality is present in a layer of Descemet’s membrane that forms around six months gestation.29 Researchers have identified two subtypes, one affecting a large area and the other affecting localized central or paracentral areas of the posterior cornea.30 The latter form is more often associated with stromal scarring.
Because the anterior surface of the cornea does not change, irregular astigmatism is less extreme than in other types of keratoconus. When scarring is present, deprivation amblyopia is a concern and surgical intervention may be necessary. In rare cases, the condition can be acquired and associated with trauma and interstitial keratitis. Because it affects the posterior cornea, treatment with rigid gas permeable contact lenses is less likely to be successful.
Terrien’s marginal degeneration. Initially, this condition affects the superior peripheral cornea asymmetrically and causes progressive thinning.31 It’s associated with fine superficial neovascularization that crosses over the thin zone, subepithelial opacification and lipid deposition leaving a clear area between the affected cornea and the limbus.31
Thinning can slowly progress to affect the circumference of the cornea with a leading edge of lipid and a central steep edge and peripheral sloping edge as further hallmarks of the thin zone. Peripheral thinning in TMD can be differentiated from furrow degeneration by the absence of progression and vessels. It can be differentiated from Mooren’s ulcer, which is characterized by pain, inflammation and an epithelial defect, as well as an absence of lipid.31
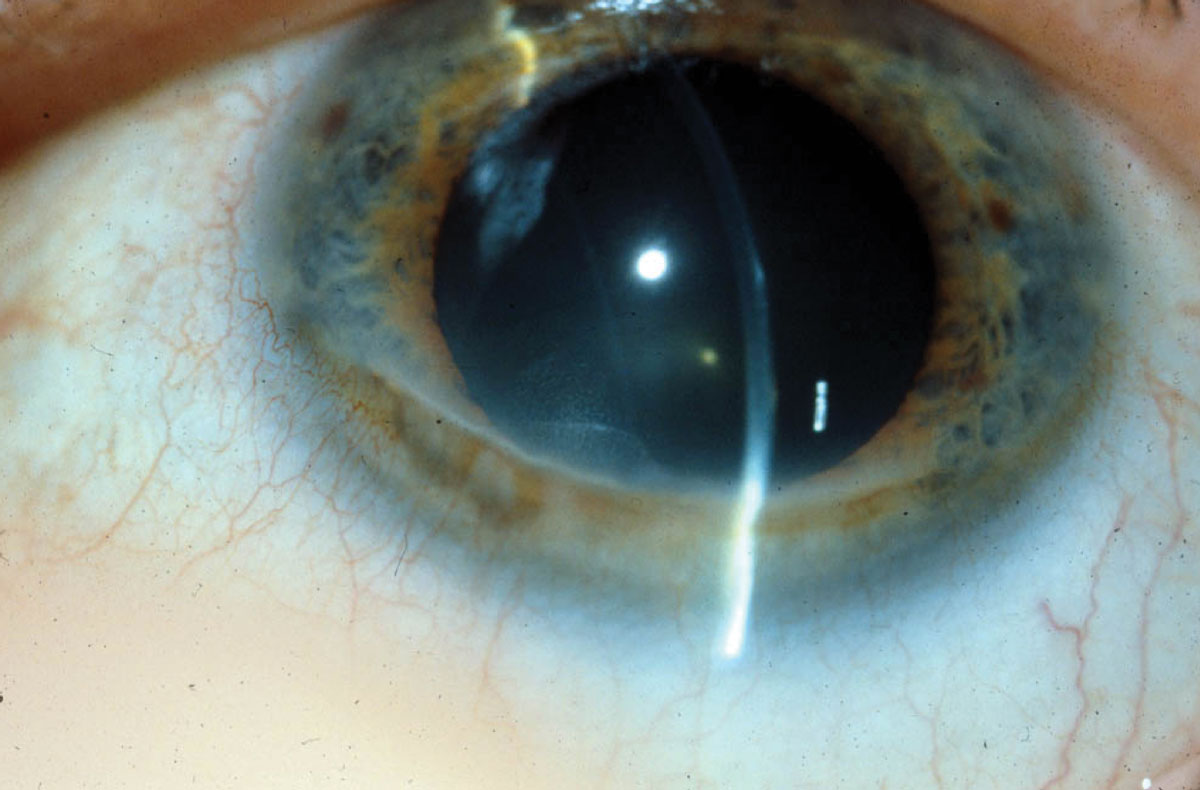 |
| In Terrien’s marginal degeneration, a clear zone of thinning with thin blood vessels crossing through is present. Opacification is associated with the steep, more central edge. Photo: Jonathan Lass, MD. Click image to enlarge. |
Like PMD, TMD is more common in men between the ages of 20 and 40 who present with increasing amounts of against-the-rule or oblique astigmatism.31
While no systemic diseases are known to be associated with TMD, the histopathological presence of large numbers of vacuoles in the affected corneal stroma may merit further study.
Most patients with TMD see well with glasses or rigid contact lenses. When corneal thickness is below 150μm and spontaneous perforation is possible, surgical options—such as tectonic grafting and lamellar keratoplasty—should be considered.31
Treatments
A thorough review of treatment for non-inflammatory irregular astigmatism is beyond the scope of this article, but options include the use of rigid lenses (corneal gas permeables, sclerals and hybrids), as well as procedures such as corneal collagen crosslinking, conductive keratoplasty, intracorneal ring implants and corneal transplants.
Non-inflammatory corneal thinning resulting in irregular astigmatism demands swift diagnosis—preferably at a stage when no slit-lamp findings are present—and expedient intervention. Of the surgical treatments available, corneal crosslinking may have the most promise as it can be performed early in the disease to arrest progression to later stages, which are harder to treat.
Dr. Stokkermans is an assistant professor at Case Western Reserve University’s Department of Ophthalmology and Visual Sciences and director of the Optometry Service at the University Hospitals Eye Institute, Cleveland, Ohio. He has a busy medical and specialty contact lens practice and has participated in multiple clinical trials, including the Collaborative Longitudinal Evaluation of Keratoconus and the National Eye Institute Refractive Error Correction Questionnaire studies.
|
1. Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Cont Lens Anterior Eye. 2010;33(4):157-66. 2. Yackle K, Fitzgerald DE. Emmetropization: An Overview. J Behav Optometry. 1999;10(2):38-42. 3. Gwiazda J, Thorn F, Bauer J, Held R. Emmetropization and the progression of manifest refraction in children followed from infancy to puberty. Clin Vision Sci. 1993;8:337-44. 4. Zadnik K, Sinnott LT, Cotter SA, et al; Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study Group. Prediction of juvenile-onset myopia. JAMA Ophthalmol. 2015;133(6):683-9. 5. Grosvenor T, Perrigin DM, Perrigin J, Maslovitz B. Houston Myopia Control Study: a randomized clinical trial. Part II. Final report by the patient care team. Am J Optom Physiol Opt. 1987;64(7):482-98. 6. Hashemi H, Fotouhi A, Yekta A, et al. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J Curr Ophthalmol. 2017;30(1):3-22. 7. Sharif-ul-Hasan K, Ansari MZH, Ali A, et al. Relative distribution and amount of different types of astigmatism in mixed ethnic population of Karachi. Pak J Ophthalmol. 2009;25(1):1-7. 8. McKendrick AM, Brennan NA. Distribution of astigmatism in the adult population. J Opt Soc Am A Opt Image Sci Vis. 1996;13(2):206-14. 9. Asharlous A, Khabazkhoob M, Yekta A, Hashemi H. Comprehensive profile of bilateral astigmatism: rule similarity and symmetry patterns of the axes in the fellow eyes. Ophthalmic Physiol Opt. 2017;37(1):33-41. 10. Read SA, Collins MJ, Carney LG. A review of astigmatism and its possible genesis. Clin Exp Optom. 2007;90(1):5-19. 11. Gordon-Shaag A, Millodot M, Ifrah R, Shneor E. Aberrations and topography in normal, keratoconus-suspect, and keratoconic eyes. Optom Vis Sci. 2012;89(4):411-8. 12. Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42(4):297-319. 13. Wheeler J, Hauser MA, Afshari NA, et al. The genetics of keratoconus: a review. Reprod Syst Sex Disord. 2012;Suppl 6. pii:001. 14. Szczotka-Flynn L, Slaughter M, McMahon T, et al; CLEK Study Group. Disease severity and family history in keratoconus. Br J Ophthalmol. 2008;92(8):1108-11. 15. Wagner H, Barr JT, Zadnik K. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: methods and findings to date. Cont Lens Anterior Eye. 2007;30(4):223-32. 16. Maeda N. Optical coherence tomography for corneal diseases. Eye Contact Lens. 2010;36(5):254-9. 17. Krumeich JH, Daniel J, Knülle A. Live-epikeratophakia for keratoconus. J Cataract Refract Surg. 1998;24(4):456-63. 18. Rabinowitz YS, McDonnell PJ. Computer-assisted corneal topography in keratoconus. Refract Corneal Surg. 1989;5(6):400-8. 19. Liu Z, Huang AJ, Pflugfelder SC. Evaluation of corneal thickness and topography in normal eyes using the Orbscan corneal topography system. Br J Ophthalmol. 1999;83(7):774-8. 20. Kent C. Catching keratoconus: Making the tough calls. Rev Ophthalmol. 2010 Jul;17(7). 21. Barr JT, Wilson BS, Gordon MO, et al; CLEK Study Group. Estimation of the incidence and factors predictive of corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea. 2006;25(1):16-25. 22. Tuft SJ, Moodaley LC, Gregory WM. Prognostic factors of progression to keratoconus. Ophthalmology. 1994;101(3):439-47. 23. Martínez-Abad A, Piñero DP. Pellucid marginal degeneration: Detection, discrimination from other corneal ectatic disorders and progression. Cont Lens Anterior Eye. November 22, 2018 Nov 22. [Epub ahead of print]. 24. Koc M, Tekin K, Inanc M, et al. Crab claw pattern on corneal topography: pellucid marginal degeneration or inferior keratoconus? Eye. 2018;32(1):11-18. 25. Mamoosa B, Razmjoo H, Peyman A, et al. Short-term result of collagen crosslinking in pellucid marginal degeneration. Adv Biomed Res. 2016 Dec;5:194. 26. Wallang BS, Das S. Keratoglobus. Eye (Lond). 2013;27(9):1004-12. 27. Silas MR, Hilkert SM, Reidy JJ, Farooq AV. Posterior keratoconus. Br J Ophthalmol. 2018;102(7):863-67. 28. Malik TG, Khalil M, Bhatti M. Topographic interpretation of posterior keratoconus. Pak J Ophthalmol. 2016;32(3):186-89. 29. Krachmer JH, Rodrigues MM. Posterior keratoconus. Arch Ophthalmol. 1978;96(10):1867-73. 30. Rao SK, Padmanabhan P. Posterior keratoconus. An expanded classification scheme based on corneal topography. Ophthalmology. 1998;105(7):1206-12. 31. Ding Y, Murri MS, Birdsong OC, et al. Terrien marginal degeneration. Surv Ophthalmol. 2019;64(2):162-74. |


