Transplantation for Limbal Stem Cell Deficiency
When severe disease calls for surgery, here’s how you can be prepared for the pre- and post-op care.
By Cecelia Koetting, OD
 |
Release Date: February 15, 2019
Expiration Date: February 15, 2022
Estimated time to complete activity: 1 hour
Jointly provided by Postgraduate Institute for Medicine and RGVCE
Educational Objectives: After completing this activity, the participant should be better able to:
- Discuss the needs and reasons for stem cell replacement.
- Identify appropriate candidates for limbal stem cell transplantation.
- Describe the current process (and a bit of the history) of harvesting and growing limbal stem cells.
- Recognize the pathophysiology of limbal stem cell diseases.
- Explain the most up-to-date procedure(s) for limbal stem cell transplantation.
- Discuss the comanagement of patients who received limbal stem cell allograft.
Target Audience: This activity is intended for optometrists engaged in the care of patients with limbal stem cell diseases.
Accreditation Statement: In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and RGVCE. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education, the Accreditation Council for Pharmacy Education, and the American Nurses Credentialing Center, to provide continuing education for the healthcare team. Postgraduate Institute for Medicine is accredited by COPE to provide continuing education to optometrists.
Faculty/Editorial Board: Cecelia Koetting, OD, referral optometric care and externship program coordinator at Virginia Eye Consultants in Norfolk, VA.
Credit Statement: This course is COPE approved for 1 hour of CE credit. Course ID is 60688-PO. Check with your local state licensing board to see if this counts toward your CE requirement for relicensure.
Disclosure Statements:
Dr. Koetting: Nothing to disclose.
Managers and Editorial Staff: The PIM planners and managers have nothing to disclose. The RGVCE planners, managers and editorial staff have nothing to disclose.
Corneal diseases, and our understanding of them, have advanced significantly over the last 15 to 20 years. One such disorder is limbal stem cell deficiency (LSCD). Diagnosis of patients with LSCD has greatly increased with technological advancements of corneal topography/tomography and anterior segment optical coherence tomography (AS-OCT). This has led to the need to improve treatment options for LSCD.
As optometrists, we are more familiar with non-surgical treatment options for LSCD, but it is also important for us to understand the surgical treatments and how to care for these patients postoperatively.
How LSCD Occurs
The cornea is comprised of six layers: epithelium, Bowman’s layer, stroma, Descemet’s membrane, Dua’s layer and the endothelium. The epithelium—the outermost layer of the cornea—is only five to seven cells thick (about 50µm).1 Peripherally, the cornea is continuous with the conjunctiva and sclera with a 0.5mm to 1mm band of limbal stem cells at the corneoscleral junction.
Limbal stem cells, which are continually shed into the tear film, are important for the regeneration of the entire corneal epithelium.1 The limbal stem cells produce the basal cell layer of the epithelium.1 These basal cells migrate towards the center of the cornea in the basal layer, moving up to become wing cells and then to become surface cells, allowing for the continued rejuvenation of the corneal surface.1 This process of corneal epithelium turnover takes approximately seven days.1
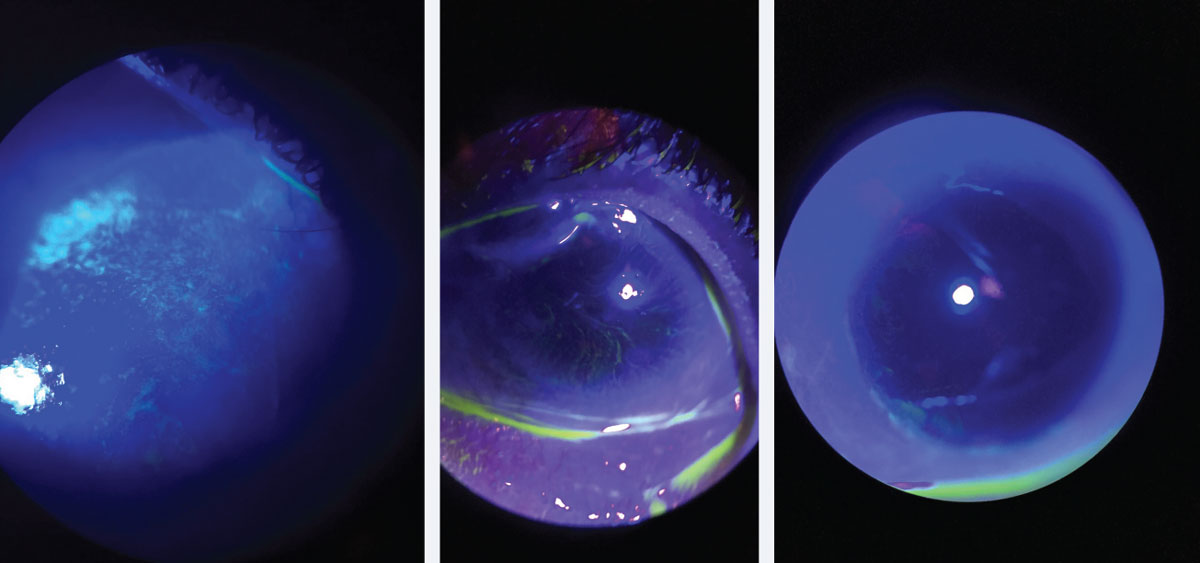 |
| Fig. 1. At left, corneal staining in patient with early LSCD. Note the stippled, elongated, almost pill like shape to the areas of staining. Middle, traditional whorl pattern staining with fluorescein. Note that the pattern starts at the limbus and begins to circle towards the apex of the cornea. At right, subtle late staining inferior and temporal with fluorescein of conjunctival cells on cornea in patient with LSCD. Click image to enlarge. |
Not only are the limbal stem cells responsible for the regeneration of the corneal epithelium, but they also create a barrier to prevent conjunctival epithelial cells from migrating to the corneal surface.2
As you can imagine, damage to or dysfunction of these limbal stem cells can be devastating to the health and function of the cornea. In LSCD, the poorly functioning limbal stem cells are unable to properly regenerate the epithelial layer, resulting in replacement by conjunctival goblet cells.3
Underlying Causes
Limbal stem cell deficiency can be either congenital or acquired, although it is most often considered an acquired condition. Congenital causes of LSCD include aniridia and ectodermal dysplasia.4
Acquired LSCD can occur from trauma due to alkaline and acidic injuries, contact lens wear, thermal injury or iatrogenic causes.4 Inflammatory issues such as chronic limbitis and bullous keratopathy and neurotrophic keratopathy from trigeminal neuralgia, diabetes mellitus, herpes simplex or herpes zoster may also lead to LSCD.4,5 Acquired LSCD can also be related to autoimmune disorders such as Stevens-Johnson syndrome and mucous membrane pemphigoid.4
Diagnosis
Patients with LSCD present with varying degrees of ocular signs, depending on the severity and level of corneal conjunctivalization. Symptoms may include decreased vision, photophobia, tearing, blepharospasm and recurrent pain.6 They may present with superficial punctate late fluorescein stippled staining at the limbus, whorl-like epitheliopathy (Figure 1), progressive ingrowth of opaque epithelium and superficial neovascularization.7 The conjunctivalized corneal surface stains abnormally because the conjunctival epithelium that has grown on the cornea is more permeable to the stain than true corneal epithelium.6
Patients with more severe disease may present with recurrent or non-healing epithelial defects, stromal scarring, or melting.7 As the cornea decompensates, the patient may experience more intense pain and loss of vision.
| Clinical Pearl Corneal punctate staining in patients with dry eye disease (DED) appears differently from the punctate staining found in LSCD patients. The staining in LSCD appears more elongated and pill shaped. Early on, the staining will be concentrated more peripheral near the limbal area. As the disease progresses and worsens, the staining will spread centrally and become more diffuse. As the natural migration of the epithelial cells to the center, the whorl-like pattern will be noted spiraling from the limbus centrally to the apex of the cornea. |
Non-surgical Treatment
The overall goal in managing patients is to prevent disease progression. So, regardless of what stage of LSCD the patient presents with, management begins with addressing the underlying etiology, which is most often inflammatory in nature. Many ocular medications and over-the-counter artificial tears contain preservatives, such as benzalkonium chloride, which can cause or exacerbate inflammation. Removing these offending agents and replacing them with preservative-free alternatives is a good start to treating these patients.
Immediate use of topical steroids can calm the reaction, although the intent is to eventually take patients off of these, if possible. Concurrently, the patient can be started on long-term use of ocular cyclosporine, which may help stabilize LSCD symptoms.8,9 Use of amniotic membrane grafts, or long-term amniotic membrane drops, helps to restore the function of limbal stem cells that may only be “stunned” in patients with mild to moderate LSCD.10
Discontinuation of contact lens wear or refitting patients into scleral lenses, which minimize trauma to the limbal area, can help eliminate mechanical rubbing and further corneal damage.11,12 Both of these options have the potential to stop or reverse early acquired LSCD.11,12
Surgical Treatment
The surgical journey of stem cell transplantation started in 1984 with keratoepithelioplasty using cadaver corneas in an allograft procedure, which was also used later in 1994 in a variation of keratolimbal allograft.13,14 This was followed shortly thereafter in 1989 with limbal autograft transplantation, which involved harvesting conjunctival and limbal tissue from the patient’s unaffected eye.13,15 By 1995, an early version of the living-related conjunctival limbal allograft technique used donor limbal and conjunctival grafts from any living relative.13,16
Here are the common surgical transplantation methods, in order of their development:
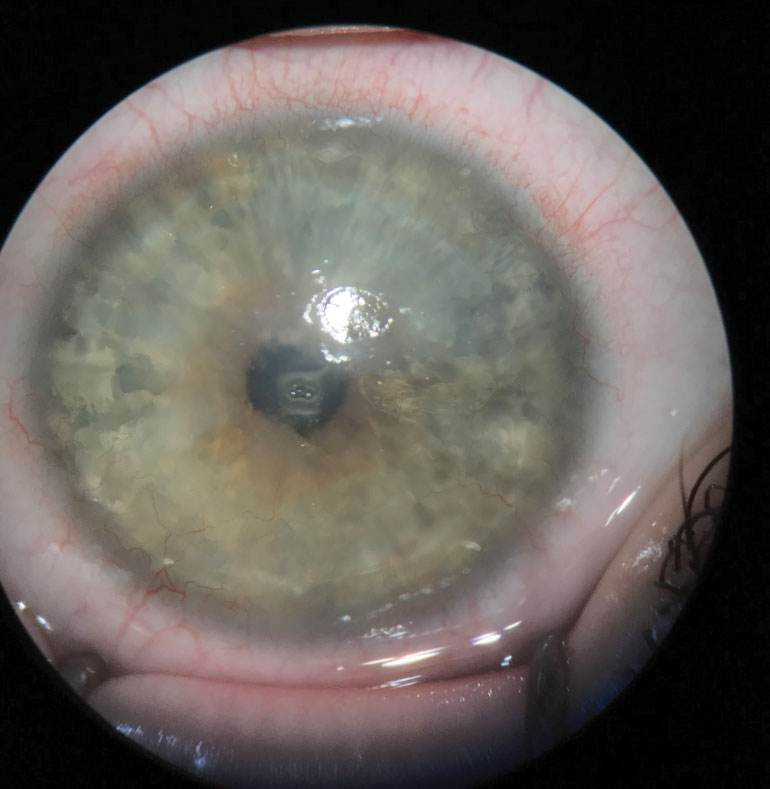 |
| Fig. 2. A moderate LSCD patient pre-operative for Ir-CLAL. Note the dullness and irregularity of the central cornea where the epithelium has begun to be replaced with conjunctival cells. Click image to enlarge. |
Keratolimbal allograft (KLAL) uses allogeneic limbal tissue attached to a corneoscleral carrier from a cadaveric donor. This procedure can be indicated for patients who have either bilateral LSCD without a related donor or a unilateral LSCD whose contralateral eye is not a viable donor.17 KLAL can be used in patients with LSCD caused by aniridia, contact lens wear or iatrogenic event with primarily limbal damage. Severe conjunctival involvement decreases the likelihood of success. For instance, conjunctiva that has chronic inflammation or scarring will have decreased mucin and tear production, creating an increased likelihood for keratinization of the ocular surface.13,14
Other etiologies of LSCD, such as mucous membrane pemphigoid and Stevens-Johnson syndrome, can be treated with KLAL but require that the eye remain controlled and quiet for at least a year prior to surgery.
Conjunctival limbal autograft (CLAU) makes use of the conjunctiva and limbus from the unaffected eye of a patient with LSCD. Up to 40% of the stem cells can be harvested from the donor eye without risk, and no systemic immunosuppression is needed.13,18
This procedure is overall extremely successful at 80% to 100% with between 25% and 100% improvement in visual acuity.12,13 The rejection rate of these grafts is minimal, with 76% at three years and 62% at six years after surgery.13,18
Living-related conjunctival limbal allograft (LR-CLAL) uses harvested limbal and conjunctival tissue from the patient’s living relative.13 It can be used in patients with both unilateral and bilateral LSCD, harvesting up to 40% of the donor’s limbal tissue with no risk to the donor (Figures 2 and 3).13 This procedure harvests considerable amounts of conjunctiva along with the limbal tissue and is well-suited for patients with significant conjunctival deficiency such as Stevens-Johnson syndrome, mucous membrane pemphigoid and chemical injuries.13 Failure risk is higher in this procedure since it is an allograft, so it requires long-term systemic immunosuppression.13
Combined conjunctival limbal and KLAL (C-CL/KLAL or C-KLAL) uses cadaveric keratolimbal tissue with LR-CLAL, helping to treat severe LSCD patients.4,13 By combining the procedures, more conjunctiva is available that is lacking in KLAL alone and increased limbal tissue that is lacking in LR-CLAL alone. Patients whose condition is severe enough to require C-KLAL may also need restoration of the conjunctival fornices and possible mucous membrane grafting. Because of this, these patients are often evaluated by oculoplastic specialists before proceeding.
Cultured limbal epithelial transplantation (CLET) is the most commonly used ex vivo method for limbal stem cell transplantation. Cultivated limbal stem cells are harvested and grown on a substrate to be later implanted into the patient’s cornea.4 The stem cells are harvested from either the patient’s fellow eye, a cadaver or a living relative.4 Oral mucosa may also be used as a tissue source.
In autologous CLET, a 2mmx2mm piece of limbal stem cell tissue is excised from the superior limbus in the patient’s healthy eye, 1mm from either side of the corneoscleral junction.4,13,18 The limbal tissue is then immediately cultured and seeded in one of multiple culture mediums.4,13,18 After the cell-seeded culture plates are allowed to grow for two to three weeks, the surgeon then implants two to three sheets of the cells in the patient’s diseased eye.4,13
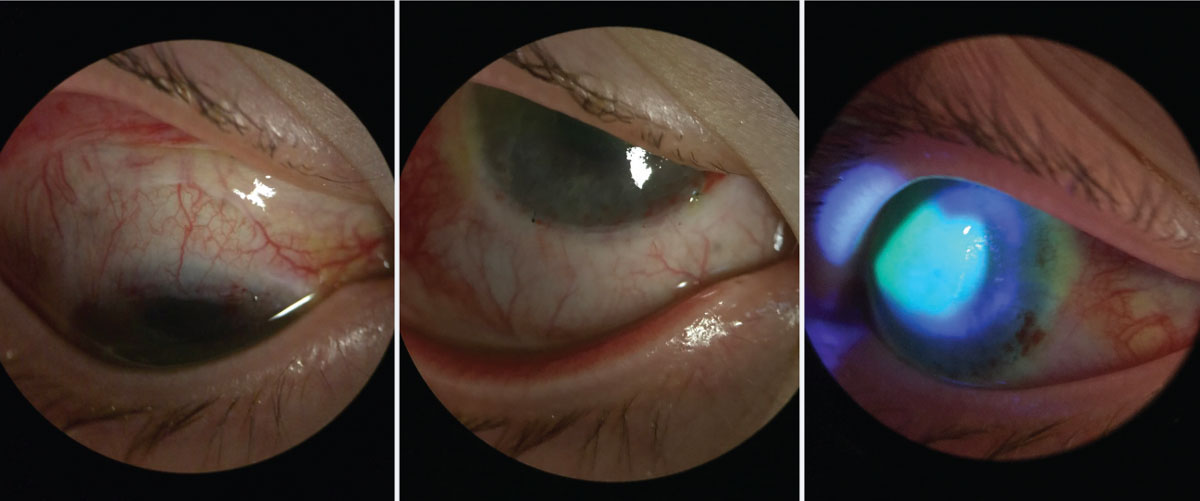 |
| Fig. 3. Same patient from Figure 4, one week postoperative after Ir-CLAL. You can see the recessed conjunctiva superior and inferior along the limbus. The patient’s central cornea has stain pooling as it continues to re-epithelialize, but there is no peripheral late staining. Click image to enlarge. |
Studies of eyes having undergone CLET have reported success rates between 67% and 77%, and yielded visual acuity improvement results in 51% to 75% of patients.10,18-21 Patients undergoing allografts vs. autografts had comparable success rates.19,20 Overall, CLET has a low complication rate, with living donor grafts having the highest safety.18
Simple limbal epithelial transplantation (SLET) is a newer technique first reported in 2012 to treat unilateral LSCD. In SLET, a 2mm-by-2mm donor limbal tissue is divided into eight to 15 small pieces and then distributed evenly over an amniotic membrane glued to the recipient’s cornea.18 Reports show success rates at anywhere from 50% to 100% with up to 75% improvement in two-line visual acuity.18 Failure rate is low and tends to occur within the first six months in higher risk patients (i.e., pre-existing symblepharon or penetrating keratoplasty).18 The most common complication after the procedure is localized reoccurrence of LSCD, which can be treated with a repeat SLET.18
Long-term Management
Immunosuppression and control of inflammation both pre- and postoperatively is key to achieving successful outcomes. Following the current Cincinnati Eye Institute protocol, prior to surgery patients are prescribed oral prednisone 1mg/kg/day and tapered over one to three months, oral valganciclovir 225mg QD, trimethoprim/sulfamethoxazole (TMP/SMX) every MWF, oral tacrolimus 4mg BID, and oral mycophenolate mofetil (MMF) 1g BID.22 Valganciclovir and TMP/SMX are stopped at 12 months.22 Tacrolimus is tapered after six months and MMF after 12 months.22
All medications are discontinued at approximately three years, but patients should continue to be monitored closely for possible rejection.22 This systemic protocol used over the last 10 years has shown to be safe and effective with minimal side effects and severe adverse events in only about 1.5% of the treated population.22
Although LSCD has been recognized as a corneal disease process for quite some time, it is becoming diagnosed and treated with increasing frequency. As clinicians, we must not only recognize the diagnosis early but also understand how it can be surgically treated and managed in severe stages.
Dr. Koetting is the referral optometric care and externship program coordinator at Virginia Eye Consultants in Norfolk, VA. She is a fellow of the American Academy of Optometry and a trustee of the Virginia Optometric Association.
|
1. Remington LA. Clinical Anatomy of the Visual System. 2nd ed. St. Louis, MO: Elsevier; 2005 2. Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol. 2000;44(5):415-25. 3. Biber JM, Holland EJ, Neff KD. Management of ocular stem cell disease. Int Ophthalmol Clin. 2010;50(3):25-34. 4. Mannis MJ, Holland EJ. Cornea: Fundamentals, Diagnosis and Management. 4th ed. Philadelphia: Elsevier; 2017. 5. Atallah MR, Palioura S, Perez VL, Amescua G. Limbal stem cell transplantation: current perspectives. Clin Ophthalmol. 2016 Apr 1;10:593-602. 6. Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48(2):83-92. 7. Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014;121(10):2053-8. 8. Baradaran-Rafii A, Javadi MA, Rezaei Kanavi M, et al. Limbal stem cell deficiency in chronic and delayed-onset mustard gas keratopathy. Ophthalmology. 2010;117(2):246-52. 9. Jadidi K, Ebrahimi A, Panahi Y, et al. Topical cyclosporine A for mustard gas induced ocular surface disorders. J Ophthalmic Vis Res. 2015;10(1):21-5. 10. Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015;34(5):592-600. 11. Schornack MM. Limbal stem cell disease: management with scleral lenses. Clin Exp Optom. 2011;94(6):592-4. 12. Jeng BH, Halfpenny CP, Meisler DM, Stock EL. Management of focal limbal stem cell deficiency associated with soft contact lens wear. Cornea. 2011;30(1):18-23. 13. Holland EJ. Management of limbal stem cell deficiency: A historical perspective, past, present, and future. Cornea. 2015;34(Suppl 10):S9-15. 14. Thoft RA. Keratoepithelioplasty. Am J Ophthalmol. 1984;97(1):1-6. 15. Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96(5):709-22. 16. Kwitko S, Marinho D, Barcaro S, et al. Allograft conjunctival transplantation for bilateral ocular surface disorders. Ophthalmology. 1995;102(7):1020-5. 17. Cheung AY, Holland EJ. Keratolimbal allograft. Curr Opin Ophthalmol. 2017;28(4):377-81. 18. Yin J, Jurkunas U. Limbal stem cell transplantation and complications. Semin Ophthalmol. 2018;33(1):134-41. 19. Baylis O, Figueiredo F, Henein C, et al. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112(4):993-1002. 20. Shortt AJ, Secker GA, Notara MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52(5):483-502. 21. Haagdorens M, Van Acker SI, Van Gerwen V, et al. Limbal stem cell deficiency: Current treatment options and emerging therapies. Stem Cells Int. 2016;2016:9798374. 22. Holland EJ, Mogilishetty G, Skeens HM, et al. Systemic immunosuppression in ocular surface stem cell transplantation: results of a 10-year experience. Cornea. 2012;31(6):655-61. |


