A Deeper Look at DEWS II Making the Diagnosis with TFOS DEWS II |
Dry eye disease (DED) affects millions of people and is one of the most frequent causes of patient visits to eye care practitioners.1 Furthermore, moderate to severe forms of DED are associated with a significant amount of pain, which limits the performance of day-to-day activities, leading to poor general health and, often, depression.
To increase our understanding of DED and better serve these patients, the Tear Film & Ocular Surface Society (TFOS) conducted the TFOS Dry Eye Workshop II (TFOS DEWS II). The Workshop, comprised of 150 clinical and basic science research experts from around the world, addressed various aspects of DED, including a significant reassessment of the tear film.2
A stable tear film is an indicator of a healthy ocular surface, primarily because it provides a refracting surface for light entering the eye and creates a safe and lubricious environment for the ocular surface tissues. Because DED is characterized by significant changes to the structure and function of the tear film, clinicians must be aware of these changes and be prepared to diagnose DED in patients who present with tear film concerns.
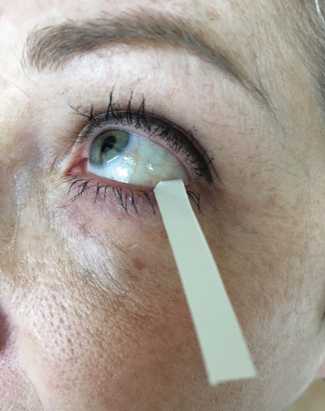 |
| Fig. 1. A Schirmer strip is placed in the lateral one-third of lower eyelid to collect a tear sample. Photo: CORE |
The TFOS DEWS II tear film report provided a detailed review of the biophysical and biochemical properties of the tears, translational dry eye tear film animal models and non-pathological factors that can impact the tear film. One of the most poignant findings of the report is the loss of tear film homeostasis as a unique characteristic of DED. Specifically, the mechanism of DED is believed to be due to evaporation-induced tear film hyperosmolarity, which damages the ocular surface by inflammation, leading to the disease. Not surprisingly, the major cause of evaporative dry eye is meibomian gland dysfunction.
This summary of the TFOS DEWS II tear film subcommittee report can help you better understand the structure and functions of the tear film, the biophysical and biochemical properties of the tear film in DED—and what it all means in your clinic.
Types and Structure
Based on the stimulus for tear production, research has broadly classified tears into four types: basal, reflex, emotional and closed-eye.3 Basal tears typically coat the eye and, in DED, are deficient. Reflex tears are produced upon stimulation of the ocular surface (e.g., vapors produced when peeling an onion) or when the reflex arc is stimulated (e.g., by nasal stimulation of the sneeze reflex). Emotional tears are also produced upon stimulation, but the stimulus here is emotions such as sadness or happiness. Closed-eye tears are those that can be collected from the ocular surface immediately after a period of sleep. Basal, reflex and emotional tears are produced mainly from the lacrimal glands via the neural arc, but differ in their protein concentration.3 The composition of closed-eye tears differs from other types as they contain an increased amount of serum-derived proteins that leak from the conjunctival blood vessels.
Historically, the tear film has been viewed as a tri-layered structure comprised of the outermost lipid, middle aqueous and an inner mucin layer. However, enough evidence has been generated over the past few years to suggest that the tear film is a bi-phasic structure composed of an outer lipid layer overlying a mucoaqueous phase.2,4
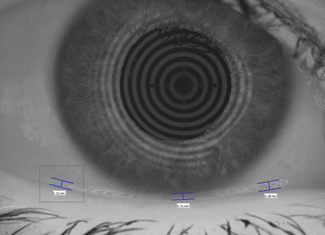 |
| Fig. 2. In this lower tear meniscus image, taken with a Keratograph 5M (Oculus), the nasal, central and temporal tear meniscus heights are indicated by the blue bars. Photo: CORE |
Biochemical Measurements in DED
The tear film lipid layer is derived from meibum secreted from the lid margins and is spread onto the tear film with each blink, driven by surface tension forces. The lipid layer is crucial for stabilizing the tear film and is thought to play a key role in preventing tear film evaporation. Studies show that the presence of only some lipids will retard tear film evaporation; thus, it may be that the lipid layer, in combination with other components of the tear film, helps to prevent water evaporation.2,5-7
The lipid layer is composed of several non-polar and amphiphilic (polar) lipids. The polar lipid family (O-acyl)-hydroxy fatty acids appear to be important in the spreading of the whole lipid layer over the muco-aqueous layer.2 However, the role of other polar lipids, such as phospholipids, is less clear. Furthermore, the TFOS DEWS II report showed that tear lipid profiles are highly variable between studies and that further investigation is warranted to understand the reason behind this variability.2
The mucoaqueous phase of the tear film is composed of at least four major mucins and more than 1,500 different proteins and peptides. Mucins are highly glycosylated proteins that help to hydrate the tears. Decreased MUC5AC expression in DED has been a consistent finding, and research suggests the deregulation of mucin synthesis can be an important factor in ocular surface disease.8-11 Several of the proteins and peptides found in the muco-aqueous phase have been shown to change with DED. Despite the fact that the protein concentration changes significantly in dry eye conditions (for more than 75 extracellular proteins and 15 intracellular proteins), no definitive set of proteins or changes in protein levels have been validated to help in diagnosis.2 Commercially available kits to detect tear film biomarkers include Inflammadry (Quidel) to test matrix metalloproteinase-9 and TearScan Lactoferrin Diagnostic Test Kit (Advanced Tear Diagnostics).
While it is widely accepted that the various components of the tear film—including lipids, proteins, mucins and salts—play a critical role in preventing tear film evaporation and collapse, the report suggested that further studies are warranted to confirm or deny this concept.
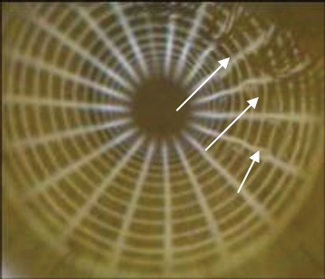 |
| Fig. 3. This image depicts NIBUT, where the arrows indicate the areas of tear break up. Photo: CORE |
Biophysical Measurements in DED
Tear production, turnover and volume can be determined using several methods; however, the correlation between these tests is limited.12 Thus, clinicians should perform a combination of tests to ensure a reliable DED diagnosis.13 The phenol red thread test is a measurement of tear volume or change in tear volume with time, wherein the clinician observes the amount of wetting of a phenol red dye-impregnated cotton thread placed over the inferior eyelid.14 A more commonly used diagnostic tool, the Schirmer test is a measure of tear production using the wetting of a standardized paper strip (Figure 1).15 Traditionally, clinicians perform the Schirmer I test without anesthesia to measure reflex tearing.
A variation of the Schirmer I test involves the use of a topical anesthetic and measures the tear basal secretion; still, a contribution from reflex tearing cannot be entirely disregarded when employing this testing variation.16 Tear clearance rate is the rate at which the tear film or an instilled marker of tears is removed from the tear film by dilution or drainage from the tear volume.17 Tear film dynamics can be assessed by dividing the Schirmer test value with anesthesia by the tear clearance rate (seldom performed in a clinical practice), which provides the tear function index.18 Research shows this value has a greater sensitivity for detecting DED than either one of these tests conducted alone.18
Tear volume, when measured using fluorophotometric methods, demonstrates a normal human tear volume of approximately 8µl ± 3µl.19 Studies show the tear meniscus height is linearly proportional to the lacrimal secretory rate, and the differences in tear meniscus height and curvature radius can be helpful in the diagnosis of DED (Figure 2).19-22 The tear film thickness, now easily measured using optical coherence tomography (OCT), ranges from 2µm to 5.5µm depending on the technique, while the lipid layer thickness ranges between 15nm and 157nm, with a mean of 42nm.23-25
 |
| Fig. 4. Tear collection for osmolarity measurement using Tearlab. Photo: CORE |
Studies show that thinning and tear film break up occur mainly due to tear film evaporation rather than the flow of fluid across the ocular surface.26,27 Some suggest the entire healthy preocular tear film resists evaporation from the ocular surface, thereby preventing tear thinning. Tear film break-up time (TBUT) measurement may provide insights into the ability of the preocular tear film to prevent evaporative losses. Clinically, although it is relatively easy to determine TBUT, interpreting the result is complicated due to its inherent variability.28,29
Several approaches exist to improve the repeatability of TBUT measurements, including taking multiple readings and averaging or selecting a subset of values or by reducing the quantity of fluorescein instilled.30-33 Whenever possible, clinicians can eliminate the use of fluorescein completely, providing a noninvasive break-up time (NIBUT) value (Figure 3). Clinicians can perform this test by projecting concentric rings (using a topographer) on the cornea and evaluating the time taken for distortions of the rings to occur. Moreover, ideal NIBUT measurements should avoid any form of tear film additives by controlling the examination environment (no additional sources of heat, air movement or humidity, for example) and adopting a standardized head posture and blink behavior.
Tear evaporation rate is considered a potential indicator of tear lipid layer stability, as increased tear evaporation rates are associated with increased tear thinning, ocular dryness and discomfort.34-37 Because of this, research suggests inter-blink tear evaporation may contribute to dry eye. Since no commercially available instrument exists, researchers have custom-built or modified instruments to determine tear evaporation rates.
Tear film osmolarity is a newer measurement that can provide insight into the balance between tear production, evaporation, drainage and absorption.38 The osmolarity of the normal tear film is determined by the concentration of electrolytes in the mucoaqueous layer. Historically, tear osmolarity was measured using freezing point depression or vapor pressure osmometry; however, their clinical utility was limited.39,40
With the introduction of a new osmometer (TearLab), clinical evaluation of tear osmolarity has increased significantly (Figure 4). This instrument uses a 50nL tear sample to analyze the electrical impedance. Mean tear film osmolarity values in normal participants range between 270mOsm/L and 315mOsm/L, with an overall average of 300mOsm/L.41-43
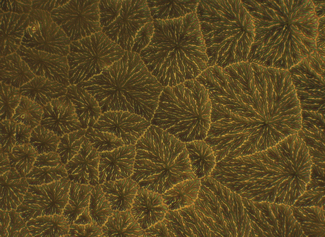 |
| Fig. 5. This image illustrates the tear ferning pattern in a normal individual. Photo: CORE |
Although the current method of tear collection involves collecting tears from the lower meniscus, clinicians should remember that the osmolarity across the ocular surface is different to that measured in the tear meniscus.44 Further studies are warranted to help establish consistent cut-off values for DED, achieved by studying a cohort where osmolarity is not a diagnostic criterion and where the sensitivity and specificity are tested in an independent study population.
Researchers have also proposed tear ferning pattern assessment as a simple and economical test for dry eye diagnosis.42,45,46 In this procedure, 1µl to 4µl of tears is placed on a glass slide, allowed to dry at room temperature (20°C to 26°C) and humidity (<50%). This slide is then observed using white light microscopy and photos are taken within 10 to 15 minutes.
In normal individuals, ferning patterns appear dense and uniform with closely branching ferns (Figure 5). However, they change with altered tear functionality and chemical composition; for example, in dry eye tears, ferning is less regular, with increased space between ferns and fern shortening, culminating in an absence of ferning altogether. Research shows tear ferning patterns are independent of sex, hormonal fluctuations in women with normal menstrual cycles and time of day during waking.2
When a tear function abnormality exists, the tear ferning patterns degrade, creating altered patterns seen in contact lens wearers and with increasing age, independent of dry eye status. Despite these promising results, more studies are needed to establish sensitivity, specificity and cut-off values in various forms of dry eye before this method can be adopted by clinicians on a regular basis.
The tear film is immensely important for the maintenance of a healthy ocular surface, and many changes occur to both the biochemical and biophysical properties of the tear film in DED. While further work will help us better characterize the biochemistry of the tear film and the role of tear film osmolarity, inflammatory markers and other biophysical properties over the entire ocular surface, much can be done now for patients. With the right knowledge and diagnostic tools, clinicians can monitor tear film changes that are harbingers of DED and initiate or alter treatment to help bring patients the stable tear film they need.
Dr. Subbaraman is a research associate professor, head of biosciences and senior clinical scientist at the Centre for Ocular Research & Education (CORE) and the School of Optometry & Vision Science at the University of Waterloo in Waterloo, ON, Canada.
1. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15:334-65. |


