Despite today’s improved contact lens care technology fueled by decades of research and the myriad of options currently available, there is still work to be done. Contact lens dropout rates due to discomfort are still an issue, and as a result, finding the right approach to best suit our patients can be difficult.1
Ralph Stone, PhD, had a long career leading teams in the development of multipurpose solutions, as well as creating an FDA classification system of lens materials for testing lens-solution interactions. Dr. Stone is currently president of RP Stone Consulting and continues as a member of the American National Standards Institute.
Contact lens safety continues to be an important consideration in today’s optometric world, and we discussed with Dr. Stone how the concerns of the past have evolved into the challenges of the future:
CS: When did you start in the contact lens industry, and what were the lens cleaning solutions of the time?
RS: I started in the contact lens and lens care industry in 1981, 10 years after the initial introduction of soft contact lenses into the United States in 1971. By that time, the primary mode of lens care was the use of heat units designed to disinfect lenses at above 80oC for at least 10 minutes. This was based on a number of experiments with a range of microorganisms. Cold disinfection solutions were introduced based on chlorhexidine and thimerosal. Both of these systems were only for disinfection, and separate enzymatic cleaners were used in conjunction with disinfection. Separate cleaners were necessary, since the life of a lens was expected to be a year, primarily because of the cost of the lens. Shortly after I began my career, hydrogen peroxide was introduced. This used the ever-present platinum disk, but also a faster, two-step system: first, soaking 3% hydrogen peroxide for at least 20 minutes, followed by a chemical neutralizer such as thiosulfate or catalase.
As time progressed, the introduction of novel cold disinfection and multipurpose solutions evolved, initially with single biocides and later with multiple active ingredients.
During this evolution, the standards for efficacy evolved as we began to understand the risks associated with contact lens wear. This did not seem to affect the rates of microbial keratitis (MK), however.
CS: Good points. Thimerosol, a mercury and thiosalicylate-based compound, causes a delayed hypersensitivity reaction in approximately 10% of people.2,3 According to the North American Contact Dermatitis Group, it is the 5th most common allergen.2,3 Also, nearly one million clinic visits are made yearly for MK, with contact lens wear being the single biggest risk factor.4 Specific issues include overnight lens wear and inadequate replacement, cleaning and disinfection of contact lenses and lens cases.4
What is the history of the contact lens classification system?
RS: The classification system (Tables 1 and 2) came as a result of the changing materials used in the contact lenses. For most of the first 10 years, all the materials had water contents less than 50%, primarily based on the use of HEMA. As companies looked to expand the offerings and recognized that we needed to get more oxygen through the lens, the water content was increased by using acids such as methacrylic acid or other polymers with better water content based on polyvinyl pyrrolidone. Those of us working on keeping lenses clean found the association of proteins with lenses was remarkably different with these newer, higher-water, acid-containing materials.
At about the same time, we started seeing that the disinfecting compounds in use were being absorbed into the lenses, creating clinical issues. In investigating these properties, we found that some clear divisions existed in the lens properties based on water content and concentration of ionic compounds in the lens materials. This was based on descriptions of how water incorporated into the polymers. The classification system was proposed and vetted throughout the industry for the testing of care systems before acceptance first in the United States and then Europe as the early descriptors of low-, medium- and high-water materials did not provide adequate ability to communicate the properties of the lenses.
With the advent of silicone hydrogel around the year 2000, we combined the properties of the rigid gas permeable materials and hydrogel materials. While that solved the issues of providing enhanced oxygen to the cornea, these lenses had all the issues related to the chemistry of both types of materials. From early on, we recognized this was a new entity, and as early as 2002 many of us were commenting on these differences in lectures and publications. The American National Standards Institute (ANSI) and the International Organization for Standardization (ISO) then put this information into systems, leading initially to the recognition of a new group: Class V materials (silicone hydrogels). As we have had increased numbers of developments using these materials, they have been divided into three subclasses, VA, VB and VC, based on the use of ionic ingredients and water content. Further subgrouping to account to the chemistries used to provide for improved surface wettability has also been added to the grouping process for class V materials. These have recently been recognized as a part of the ANSI and ISO standards for contact lenses.
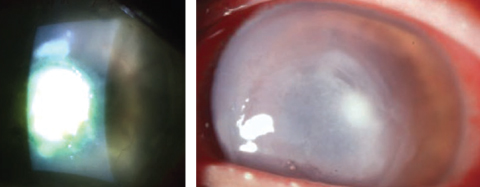 |
| Staphylococcus aureus, left, and Pseudomonas aeruginosa, right, were among the five organisms tested by the ISO to develop its recent contact lens solution disinfection testing guidelines. Photos: Scott G. Hauswirth, OD, and Richard Mangan, OD |
CS: Thanks for mapping that out for us. To expand a little on the ISO subdivision, its recent version of the contact lens solution disinfection testing guidelines includes recommendations for lens soaking and storage periods when contaminating microorganisms are introduced through patient handling.5 To develop these recommendations, the antimicrobial efficacy of the test solution was evaluated at specific time intervals of 24 hours, seven days and the maximum labeled storage as stated by the lens case manufacturer.5 Five challenge organisms were tested: Staphylococcus aureus, Pseudomonas aeruginosa, Serratia marcesens, Candida albicans and Fusarium solani.5 This standard is reviewed every five years.5
Moving on, what are the biggest misconceptions about contact lens care solutions? What do practitioners need to know to keep patients safe?
RS: The first misconception is that they produce sterile lenses with no bacteria or fungi associated with the lens or the case. The disinfecting properties are designed to reduce the level of organisms to “safe” levels. In testing, this means that when used for disinfection or in a regimen, the solution by itself reduces the presence of organisms from a million to less than 10. While this requires activity far higher than the levels observed on lenses in normal usage by a factor of a 100 to 1,000, it is not zero.
The second misconception is that lenses going back into the eye are as good as in the case at the end of the care cycle. The biggest source of contamination, other than dropping lenses, is the patient’s hands during lens handling and insertion. Studies looking at lenses taken from the eye using sterile techniques show very low levels of contamination, in the order of 100 to 200. After handling, however, lenses going into the disinfection process have levels reaching into the thousands, which is the level of disinfection the systems have to deal with. When we put a lens back on the eye, the organisms from the finger are introduced onto the lens. This is probably why we do not see a big difference in the rate of infections between daily disposable lenses and frequent replacement lenses, although severity appears to be less.
The third misconception is that the care systems are equally effective against all organisms, even the different species and strains. Although the biocides used for lens care are designed to work through mechanisms that minimize organism survival, the biocides are not equally effective in all instances, and sometimes survivors remain. This is often seen as contamination in the cases of various organisms. The simplest example, although not the only one, is hydrogen peroxide. Some organisms produce catalase, an enzyme that may inactivate peroxide, reducing its efficacy. This points to our need for regular case cleaning and replacement by patients. How many cases have we seen that are visibly dirty?
The fourth misconception is in our teaching of compliance. Overall, we don’t do a good job of this. Every major recall related to infections has been linked to “topping off.” Multipurpose solutions are designed for use with a single bottle meant to last about one month, but usage rates are only about 3.6 to 3.8 bottles a year. While we recognize the need for emptying, cleaning and refilling cases with fresh solution, patients do not do it. These solutions are designed for use for a single cycle, whether it is hydrogen peroxide, which is neutralized, or a multipurpose solution. We are not emphasizing this enough in our practices. Contact lenses are recognized as a medical device used in the eye, and patients need to understand the importance of good hygiene.
CS: Very true. A couple side notes: Overnight wear, increased exposure in daily wear, smoking and poor hand hygiene are significant risk factors for MK.6 However, the organisms isolated on the lenses are consistent with less severe disease.6 Also, the most efficacious way to clean a contact lens case is to rinse the case with multipurpose solution, followed by wiping out the case with a clean tissue and air-drying.7
Next question: What has been the biggest advance in solution knowledge during your career?
RS: I think of two areas that have enhanced the proliferation of safe contact lens wear. The first is the development of better, safer and less toxic biocides. During my career we have introduced the current array of novel biocides, starting with relying on heat and chlorhexidine/thimerosal disinfectants and advancing to hydrogen peroxide, polyhexamethylene biguanide, polyquaternium-1 and, more recently, Aldox and alexidine. We have learned that we can be even more effective by using combinations to provide a broader spectrum of activity.
The second and more difficult area has been keeping lenses wet and lubricated. We have known that soft contact lenses tend to get dry areas over time, causing the polymer to accommodate by migrating the like hydrophobic portions of the polymer chains to the surface. This dryness may be at least partially responsible for the major issue facing the field: contact lens dropouts.
We recognized early on that the eye does try to accommodate and make lenses wet. Studies focused on spoilation of extended wear lenses showed that comfort was not best on day one, but rather on days two, three and sometimes four, with a decrease going forward as the fouling became more of an issue.
Since the lid may be the primary driver in all this, practitioners have been looking for ways to keep lenses wet and lubricated. New wetting agents have helped solutions maintain their action over the course of the day. This has improved the maintenance of the lenses during care, and the introduction of silicone hydrogels has made this even more important. Lenses are often modified at the surface with bonded chemistry that minimizes the appearance of non-wetting areas.
CS: What changes still need to be made?
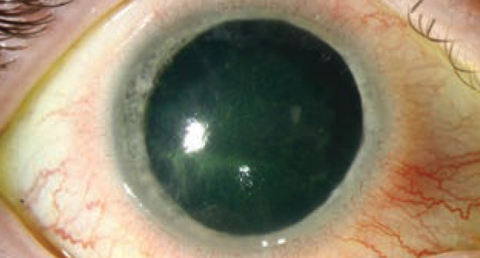 |
| While Acanthamoeba infections, shown here, aren’t the most frequently encountered of entities seen in clinical practice, they are serious events that require swift treatment. |
RS: The first and most prominent need in this industry is to make compliance a number-one priority. I recently read an article indicating the importance of reducing chair time. However, practitioners should not skip patient education just for the sake of reducing chair time. It is imperative that both doctor and staff put emphasis on teaching patients the importance of using a medical device.
The second need is development of better case technology. We still are using the same technologies developed in the 1970s and ‘80s. Significant levels of case contamination continue to be reported, even in controlled clinical trials. Development of cases that engender compliance and have finite lifetimes is critical.
While several of these concepts are on the drawing boards, few have seen the marketplace. This is needed to minimize the inoculation of organisms into the eye.
The third is to understand the occurrence of Acanthamoeba infections. These continue to occur at a low level, but are still serious events. There appears to be an increase from levels seen around 10 years earlier. While many of our current products have some activity, we are still trying to find a reproducible measurement system to understand the effectiveness.
Testing methods are currently undergoing multi-laboratory evaluations to validate these options. Researchers speculate that the increase noted in 2007 was first related to a single product and, overall, probably related to changes in our drinking water requirements.
The final need is continued exploration of biocide technologies, as clinical isolates will continue to develop that are outside the range of current efficacy standards or products. No single option or combination can be totally effective against all organisms and the multitude of strains present as they continue to evolve. It is imperative to note that testing of clinical strains outside of standard culture collections is not necessarily reproducible between laboratories, as the organisms continue to mutate. As such, we must pursue continued monitoring of the organisms causing infections.
| Table 1. Conventional Hydrophilic Material Groups | |
| Group | Description |
| I | Low Water Content (<50%), Nonionic* |
| II | High Water Content (>50%), Nonionic* |
| III | Low Water Content (<50%), Ionic* |
| III | High Water Content (>50%), Ionic* |
| *Being ionic in pH = 6.0 - 8.0 | |
| Table 2. Silicone Hydrophilic Material Groups | |
| Group | Description |
| V-A | No Water Specification Ionic* |
| V-B | High Water Content (>50%), Nonionic* |
| V-C | Low Water Content (<50%), Nonionic*, Hydrophilic-monomer Only |
| V-Cm | Low Water Content (<50%), Nonionic*, Surface Tested (ST) |
| V-Cr | Low Water Content (<50%), Nonionic(, Non-ST, Semi-interpenetrating Network |
| *Being ionic in pH = 6.0 - 8.0 | |
While the industry has made great strides in contact lens care such as improved biocides and lens wetting systems, there is still much that can be done to create a better experience for our contact lens patients. In order to continue our growth, we only need to look to our past and how it has shaped our present. Dr. Stone has seen this evolution firsthand, and looking to experience like his for guidance will be helpful as we move forward.
Dr. Sindt is the director of contact lens service and a clinical professor at the University of Iowa. She is also associate clinical editor of Review of Cornea & Contact Lenses.
1. Dumbleton K, Woods CA, Jones LW, Fonn D. The impact of contemporary contact lenses on contact lens discontinuation. Eye Contact Lens. 2013;39(1):92-98. |


