 |  |
Seen largely as instruments for the posterior segment, most clinicians and industry partners have overlooked its utility in visualizing the anterior segment. We, however, have been using OCT to view and analyze the anterior segment for years.
In this column, we review some technological advancements that are currently available or are in development, as well as postulate where the technology may go in the future. In our minds, OCT can be as much a part of our anterior segment assessment as it is for the posterior segment, and it may possibly become a daily practice mainstay similar to the tonometer or autorefractor.
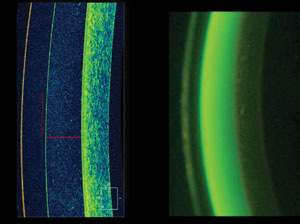 |
| Fig. 1. These images compare the detail provided by OCT measurement (left) with the variability of the estimation method (right). |
We first began using OCT instruments to measure sagittal depth of our custom scleral contact lens fits. Early to the scleral lens world in 2004, we traditionally used the estimation method to view how much clearance the lens had from the cornea. Soon, we realized we could use the anterior chamber feature of our OCT to image the lens, tear film and cornea. Although the estimation method was suitable in the past, its variability begged for a better way—leaving room for OCT, which has now become the mainstay when fitting scleral lenses.
A study comparing the estimation method with OCT use found a margin of error ranging from 53μm to 207μm. For the right eyes in particular, the difference between the observer and OCT averaged 128μm (Figure 1).1
Initially, the anterior OCT was designed to view the anterior chamber angle in patients with glaucoma. Today, the value of an anterior OCT can range far beyond glaucoma alone.
An early OCT model that gained attention in cataract and glaucoma circles was the Zeiss Visante, which produced widefield cross-section tomography of the anterior chamber. Its major benefit was the capability to produce images of the anterior chamber beyond limbus to limbus. Unfortunately, the system’s limited software capabilities and advancements stifled its adoption by the eye care market (Figure 2).
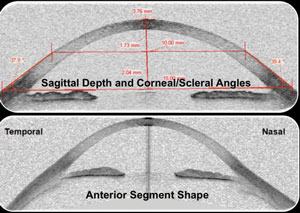 |
| Fig. 2. The Visante OCT (Carl Zeiss) provided a glimpse of widefield anterior segment imaging, but had limited software capabilities. |
Following the glimpse into the anterior chamber the Zeiss Visante provided, many OCT companies began looking for ways to expand their own anterior views. Current OCT hardware does not allow for wider-angle views of the anterior chamber. As such, all current OCT systems require the use of ancillary lenses to capture these additional views.
While newer hardware has expanded OCT imaging with much wider views, they still do not provide the full limbus-to-limbus or conjunctiva-to-conjunctiva views provided by the original Zeiss Visante. Clinicians can steer the instrument to spread the view beyond this area of the cornea, but the technology isn’t ideal. We predict this will be one of the biggest opportunities for differentiation in future OCT instruments (Figure 3).
Another area in need of improvement is progression software. Although today’s OCT instruments have reached beyond what our expectations were several years ago, we know they can do more.
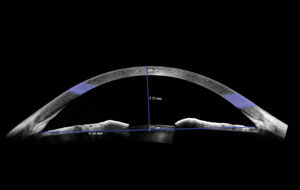 |
| Fig. 3. Modern widefield anterior segment OCT still does not provide full limbus-to-limbus or conjunctiva-to-conjunctiva views. |
Originally, we used specialized calipers to measure corneal thickness on our small-field OCT scans. Now, advanced OCT technologies allow for pachymetry maps of the cornea. The newer Zeiss instruments, for instance, can reach 9mm in diameter, and the maps can show various areas of corneal thickness at one time. As with topography, we can visualize and measure changes in patients with corneal anomalies such as keratoconus.
Corneal topography remains the mainstay for detecting and measuring keratoconus in many practices, largely because it has a greater adoption rate than anterior segment OCT or specialized instruments that measure posterior and anterior curvatures such as Orbscan (Bausch + Lomb) or Pentacam (Oculus). However, topography has its limitations, including variability when imaging the tear film and capturing highly irregular surfaces. As such, we have found topography is limited in its ability to show corneal change for advancing keratoconus patients.
OCT, on the other hand, allows us to measure thickness the central cornea. Future software developments and advanced hardware systems could help OCT become the hallmark for keratoconus and other corneal diagnoses (Figure 4).
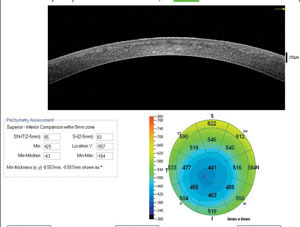 |
| Fig. 4. Pachmetry map of patient with keratoconus. |
Measure, Calculate and Treat
Other exciting advancements in OCT imaging relate to power and curvature calculations and the measurement of epithelial thickness mapping, which can help with the detection and management of corneal anomalies and dry eye (Figure 5).
Some advancements are limited to research settings or are only available outside the United States, but we hope to see them progress into clinical practice soon. We predict OCT advancements with nerve assessment will one day make it a useful tool for other corneal diseases and dry eye issues. For example, software that can map cataract density and location may help determine cataract severity and type, while length
measurements will help with intraocular lens calculations andfuture refractive error calculations from all areas of the cornea, possibly providing a better understanding of refractive error and myopia control.
Lastly, partnering point-of-care diagnostics with dynamic OCT
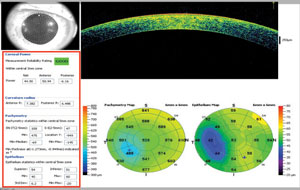 |
| Fig. 5. Power and curvature calculations can help clinicians detect and manage corneal anomalies and dry eye. |
It is time to bring OCT to the forefront of eye care. It has applications for both posterior and anterior segment assessment—far more than what we might imagine possible. It is important for us to drive our industry partners to push the limits and expand the parameters of our current technological capabilities.
1. Brujic M. Estimating scleral lens clearance and comparing it to OCT measured clearance. Poster presented at the 2015 Global Specialty Lens Symposium, January 22, 2015; Las Vegas.


