 |
However, ophthalmic eye drops are not ideal for several reasons. First, they inherently have poor bioavailability, and drug level varies from one drop to the next. Second, eye drop schedule adherence can be problematic. In fact, most patients are also instructed to begin using eye drops a few days prior to cataract surgery, further extending the burden of drop instillation. Third, they have the potential for adverse responses, including toxicity or allergic reaction to either the active ingredient or preservative, as patients are often using the steroid and NSAID for several weeks. Finally, it can be expensive to use several medications at once, resulting in substitution for generic formulations.1
The Compliance Issue
Research suggests noncompliance with glaucoma medications can be as high as 40%.2 Adherence should be better for a shorter course of therapy with the objective of preventing complications after a surgery—but it’s not. In a recent study, eyedrop-naïve patients undergoing cataract surgery were recruited at the one-day postoperative visit. They completed a questionnaire about their postoperative medication use and were videotaped using their drops. The study found a major disconnect between subjective and objective reports. According to the subjects, 69% washed their hands before instilling drops, 42% were always accurate getting the drop in their eye and 58% never contaminating the dropper by touching their eye. But the videotape, reviewed by two independent researchers, showed that 93% demonstrated an improper instillation technique, 32% missed the eye, 57% contaminated the bottle and 78% did not wash their hands. Patients who had received instruction on drop usage did perform better.3
With such poor compliance, it’s no wonder surgeons are looking for alternative methods of delivering postoperative medications including ophthalmic combination drops and injectable medications at the time of surgery.
Combination Medications
Using fewer drops means combining multiple therapeutic agents in one bottle. Pred-Gati (Imprimis Pharmaceuticals) consists of 1% prednisolone acetate and 0.5% gatifloxacin, Pred-Gati-Nepaf (Imprimis Pharmaceuticals) adds 0.1% nepafenac and Pred-Nepaf (Imprimis Pharmaceuticals) eliminates the antibiotic. All three are formulated as suspensions, available in 3mL bottles and cost $25.
These combination medications have several benefits: (1) the low cost makes it easier to prescribe different drops at different times in the postoperative regimen, (2) patients only have to use one bottle, (3) there is less exposure to preservatives, reducing the likelihood of toxicity, and (4) drug dilution is eliminated, as only one drop is instilled rather than several consecutive drops.4
Dropless
Recent studies show moxifloxacin is as safe as a balanced salt solution when injected into the anterior chamber, with no toxicity. There was no statistically significant difference in acuity, intraocular pressure (IOP), corneal integrity (including endothelial cell counts) and anterior chamber reaction between either injection.5 Investigators also show intravitreally injected triamcinolone performs similarly to topical prednisolone acetate in terms of reducing postoperative cells and flare, impact on IOP, patient symptoms and rate of complications.4 These findings set the stage for a new way of managing patients postoperatively—dropless.
Drugs injected transzonularly behave very differently, pharmacokinetically, than an intracameral injection, which is quickly washed out. A transzonular injection is retained in the vitreous matrix for an extended time, providing a sustained release of medications.4
Some surgeons are compounding their own injections. Imprimis has two injectable formulations available. Tri-Moxi contains triamcinolone acetonide and moxifloxacin. Tri-Moxi-Vanc adds vancomycin. They are injected through a cannula passed through the cataract incision site after the lens implant is in place but before the viscoelastic has been removed.6
In a chart review of 2,300 cataract-surgery eyes that received Tri-Moxi injections, there were no cases of endophthalmitis.6
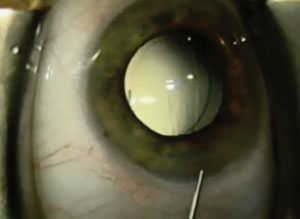 |
| During transzonular drug delivery, the characteristic white pupil is evidence of quick diffusion throughout the eye and is the endpoint of uncomplicated cataract surgery. |
One recent study compared use of a Tri-Moxi-Vanc injection with a Pred-Moxi-Ketorolac (Imprimis Pharmaceuticals) single drop combination, and then Pred-Ketor (Imprimis Pharmaceuticals). Pred-Moxi-Ketorolac was used for one week and then discontinued, while Pred-Ketor was used for three more weeks. It was a small study of 25 patients with one eye receiving each treatment, so each patient’s contralateral eye served as the control. Outcomes were similar for the two groups in terms of IOP, central macular thickness and pain. Patients did prefer the injection for an improved overall cataract surgery experience, but there was similar visual acuity for the injected group vs. the topical group.4
A new study found that, of 1,541 eyes that received an intravitreal injection of Tri-Moxi-Vanc, none developed postoperative endophthalmitis, and 92% did not require supplemental medication following cataract surgery. Nine percent had breakthrough inflammation, requiring additional anti-inflammatory agents. The rate of CME was 2% and interestingly, there was no difference in the incidence in diabetic patients compared with non-diabetics. Less than 1% of patients manifested IOP greater than 10mm Hg at two-week or three-month follow up.8
While using fewer drops is a step in the right direction for improved postoperative care, dropless cataract surgery holds numerous advantages above and beyond this treatment advancement. Transzonular injections during surgery can provide predictable delivery of medication, excellent prophylaxis against infection and simplified postoperative care. Patients do not need to purchase drops or remember to use them, and physicians do not need to explain the medication schedule. Getting rid of drops could mean more satisfied patients.8
1. Stephenson, M. New alternatives in post-cataract pharmacology. Review of Ophthalmology. Available at www.reviewofophthalmology.com/article/new-alternatives-in-postcataract-pharmacology-feb16. Accessed February 1, 2017.
2. Vandenbroeck S, De Geest S, Dobbels F, et al. Prevalence and correlates of self-reported nonadherence with eye drop treatment: the Belgian compliance study in ophthalmology. J Glaucoma. 2011 Sep;20(7):414-21.
3. An JA, Kasner O, Samek DA, Lévesque V. Evaluation of eyedrop administration by inexperienced patients after cataract surgery. J Cataract Refract Surg. 2014 Nov;40(11):1857-61.
4. Fisher BL, Potvin R. Transzonular vitreous injection vs a single drop compounded topical pharmaceutical regimen after cataract surgery. Clin Ophthalmol. 2016 Jul;10:1297-303.
5. Lane SS, Osher RH, Masket S, Belani S. Evaluation of the safety of prophylactic intracameral moxifloxacin in cataract surgery. J Cataract Refract Surg. 2008 Sep;34(9):1451-9.
6. Dalton M. Dropless cataract surgery offers ‘significant benefit.’ Ophthalmology Times. Available at www.ophthalmologytimes.modernmedicine.com/ophthalmologytimes/news/dropless-cataract-surgery-offers-significant-benefit. Accessed February 2, 2017.
7. Stringham JD, Flynn HW Jr, Schimel AM, Banta JT. Dropless cataract surgery: what are the potential downsides? Am J Ophthalmol. 2016 Apr;164:8-10.
8. Tyson SL, Bailey R, Roman JS, et al. Clinical outcomes after injection of a compounded pharmaceutical for prophylaxis after cataract surgery: a large-scale review. Curr Opin Ophthalmol. 2017 Jan;28(1):73-80.


